This is an automatically translated article.
Article by Doctor Pham Anh Tuan - Vinmec Institute of Stem Cell and Gene TechnologyAmong people with the most common type of lung cancer, up to 40% of patients develop metastatic brain tumors, with a median survival of less than six months. But why non-small cell lung cancer (NSCLC) often metastasizes to the brain remains poorly understood.
Recently, scientists at Wake Forest School of Medicine discovered that nicotine, a non-carcinogenic substance found in cigarettes, actually promotes the spread/metastasis of lung cancer cells into Brain. Professor Kounosuke Watabe, lead researcher, at Wake Forest School of Medicine said: "Based on our findings, we do not think nicotine replacement products are the safest way for people with the condition. smoking cessation lung cancer".
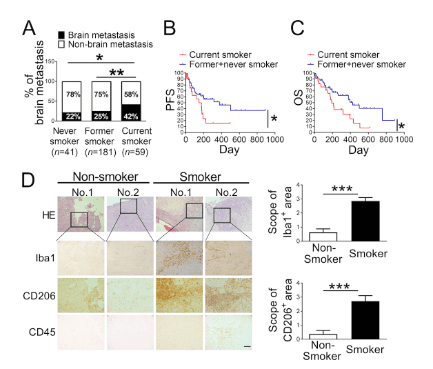
Fig.1. Cigarette smoking increases incidence and mortality rate of lung cancer brain metastasis.
(A) Incidence of brain metastasis among 281 stage IV lung cancer patients composed of both current and noncurrent smokers at the time of diagnosis. All patients were admitted to Wake Forest Baptist Hospital. (B and C) Progression-free survival (PFS; B) and overall survival (OS; C) of patients with brain metastasis ( n = 79) and with or without smoking history were examined by Kaplan–Meier analysis. (D) Representative images of immunohistochemical analysis for Iba1+, CD206+, and CD45+ cells in the H&E-stained brain metastatic lesions of lung cancer patients who were current smokers ( n = 7) and noncurrent smokers ( n = 4). Scale bar, 100 μm. *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
In the study, published June 4 in the Journal of Experimental Medicine, Professor Watabe's team first examined 281 lung cancer patients and found that smokers had a higher rate significantly higher incidence of brain cancer.
Then, using a mouse model, researchers found that nicotine enhances brain metastasis by crossing the blood-brain barrier (BBB) to alter the function of microglia - a type of immune cell in the brain - from protecting to promoting tumor growth.
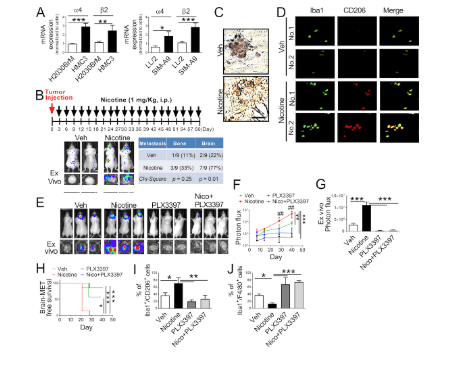
Fig.2. Nicotine promotes brain metastasis by activating microglia in vivo.
(A) Expression of α4β2 nicotine receptor on human microglia (HMC3), mouse microglia (SIM-A9), human lung cells (H2030BrM), and mouse lung cancer cells (LL/2) were evaluated by qRT-PCR (A) = 3/group). (B) LL/2 (5×104 cells) were intracardially injected into immune-competent BALB/c mice ( n = 9). After 3 d of intracardiac transplantation of LL/2 cells, mice received nicotine treatment (1 mg/kg) by intraperitoneal injection every 3 d for 60 d. Upper panel: BLI images of brain metastatic lesions of representative mice from each experimental group (vehicle alone or nicotine treatment). Lower panel: total photon flux of ex vivo image of brain metastatic lesions was measured by BLI at the end point (day 60). Quantitative data of bone and brain metastasis of lung cancer are shown in the right panel. (C) At the end point, the brain sections from mice with or without treatment with nicotine were examined for Iba1+ signal (brown) on microglia. Scale bar, 20 μm. (D) Representative images of immunohistochemical analysis for CD206+ and Iba1+ microglia in the metastatic brain lesions of mice that were treated with or without nicotine ( n = 9/group). Scale bar, 100 μm. (E) Mouse lung cancer LL/2 cells were intracranially injected into wild-type BALB/c mice ( n = 9/group) followed by administering PLX3397 by intracranial injection. Upper panels are BLI images of brain metastatic lesions of representative mice from each experimental group at day 40. Lower panels represent the total photon flux of ex vivo brain metastatic lesions as measured by BLI at the end point (day 40). (F) Quantitative data of in vivo brain metastasis of lung cancer (n = 9/group). (G) Ex vivo BLI signals in the brain at the end point (n = 9/group). (H) Kaplan–Meier analysis of brain metastasis–free survival was performed ( n = 9/group). (I and J) Metastatic brain tumors in E were isolated from the brain and were examined by FACS for M2 (I) and M1 (J) microglial polarization ( n = 4 or 5/group). The data are presented as the mean ± SD. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 versus respective nicotine group.
, P < 0.01 versus respectively PLX3397 or Nico+PLX3397 group.
Feverfew is a short-stemmed, bushy herb with a height of 0.3m to 1m in the family Asteraceae, scientific name is Tanacetum Parthenium L. Watabe and colleagues searched for drugs with can reverse the effects of nicotine and have identified parthenolide, a natural substance in this herbal antipyretic, can block nicotine-induced brain tumor metastasis in rats. Since chrysanthemum has been used for many years and is considered safe, Watabe believes parthenolide could provide a new way to combat brain metastases, especially for ex-smokers. or still smoking.
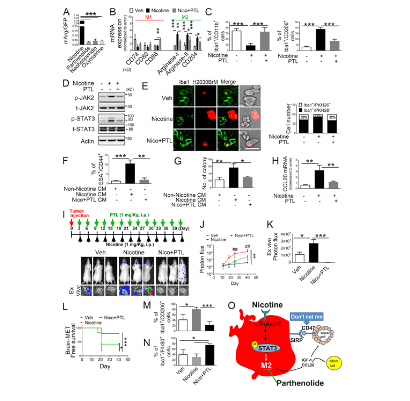
Fig.3.PTL suppresses brain tumor progression by blocking nicotine-induced M2 microglia polarization.
(A) Human microglia cells (HMC3) with the Arg1 reporter plasmid were cultured in the presence or absence of compounds that were identified as the top three most effective inhibitors for Arg1 during our initial screening (see Materials and methods). After 48 h of incubation, luciferase reporter activity was measured (n = 4/group). (B) Expression of surface markers of M1/M2 microglia was examined by qRT-PCR after microglial cells were treated with or without nicotine plus PTL (n = 4/group). (C) The same set of samples in B was evaluated for quantification of Iba1+/CD11b+ (M1) cells and Iba1+/CD206+ (M2) cells by FACS ( n = 4/group). (D) The same set of samples in C was examined for quantification of the protein expression of JAK2 and STAT3 by Western blot. (E) Human microglial (HMC3) cells (green) with or without nicotine treatment (1 μM) in the presence or absence of PTL (1 μM) were incubated with PKH26-labeled H2030BrM cells (red) for 24 h and photographed (left) panels), followed by measurement of the microglial phagocytic activity (right panel; n = 4/group). Scale bar, 10 μm. (F) CM was prepared from human microglia (HMC3) treated with or without nicotine and PTL. The CM was added to the culture of H2030BrM, and cells were incubated for 48 h followed by evaluation of CSC population by FACS. Non-nicotine, nicotine, or Nico+PTL CM: microglia were treated with PBS, nicotine, or nicotine plus PTL for 24 h. They were then washed twice with PBS and incubated in the fresh DMEM/F12 medium supplemented with 2% FBS for a further 24 h ( n = 4/group). (G) For the same set of samples as F, colony-forming ability was also measured ( n = 4/group). (H) Human microglia were treated with or without nicotine (1 μM) in the presence or absence of PTL for 24 h, followed by assessment of the expression of CCL20 by qRT-PCR ( n = 4/group). (I) The mouse lung cancer cells, LL/2, were intracardially injected into wild-type BALB/c mice. After 3 d of intracranial transplantation of LL/2 cells, mice received nicotine (1 mg/kg) plus PTL (1 mg/kg) by an intraperitoneal injection every 3 d for 40 d. Upper panel: BLI images of representative mice from each experimental group at day 40. Lower panel: total photon flux of ex vivo brain metastatic lesions was measured by BLI at the endpoint (day 40; n = 9/group). (J) Quantitative data of BLI in the brain regions are shown ( n = 9/group). (K) Ex vivo signals in the whole brains at the end point were quantified. (L) The Kaplan–Meier analysis of brain metastasis–free survival was performed ( n = 9/group). (M and N) Metastatic brain tumors in I were isolated from the brain and were examined by FACS for M2 (M) and M1 (N) microglial polarization ( n = 9/group). (O) A proposed model illustrating a nicotine-induced brain metastasis ( n = 9/group). The data are presented as the mean ± SD. *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
Currently, the only treatment for lung cancer, this devastating disease is radiation therapy. Traditional chemotherapy drugs cannot cross the blood-brain barrier, but parthenolide does. Therefore, it holds promise as a treatment or maybe even a way to prevent tumor metastasis to the brain.
The team says it will work with oncologists at Wake Forest School of Medicine, to develop a clinical trial that will test parthenolide in the near future.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References:Kounosuke Watabe, Andrew Dothard, Thomas W. Lycan, Jimmy Ruiz, Michael Chan, Wei Zhang, Tamjeed Ahmed, Yusuke Shiozawa, Ravindra Pramod Deshpande, Dan Zhao, Yin Liu, Abhishek Tyagi, Kerui Wu, Sambad Sharma , Fei Xing, Shih Ying Wu. Nicotine promotes brain metastasis by polarizing microglia and suppressing innate immune function . Journal of Experimental Medicine, 2020; 217 (8) Kakusa, B., S. Han, S. Aggarwal, B. Liu, G. Li, S. Soltys, and M. Hayden Gephart. 2018. Clinical factors associated with mortality within three months after radiosurgery of asymptomatic brain metastases from non-small cell lung cancer . J. Neurooncol. 140:705–715. Fidler, I.J.. 2015. The Biology of Brain Metastasis: Challenges for Therapy. Cancer J. 21:284–293 Bacha, S., H. Cherif, D. Rabaa, S. Habibech, S. Cheikhrouhou, H. Racil, N. Chaouch, M.L. Megdiche, and A. Chabbou. 2018. Brain metastases of non-small cell lung cancer: prognostic factors and management . Tunis. Med. 96:165–171. Saving lives of lung cancer patients with a new generation of targeted drugs