This is an automatically translated article.
Hepatitis B virus (HBV) causes hepatitis B. There are currently many drugs used in the treatment of hepatitis B, which work to kill the virus. However, the HBV virus also has mutations that are resistant to one or more of these drugs, making treatment difficult.1. HBV drug resistance mutations
Currently, more than 2 billion people worldwide are infected with hepatitis B virus (HBV), of which 350 million are chronic carriers of the hepatitis B virus. HBV infection can lead to acute or chronic necrotizing hepatitis, cirrhosis, and especially hepatocellular carcinoma (HCC), causing approximately 0.5 to 1.2 million deaths each year. five.
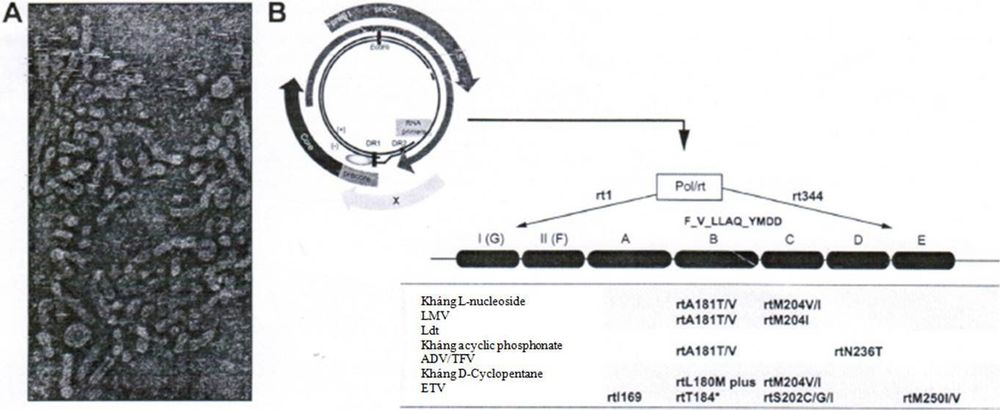
Hepatitis B virus belongs to the hepadnavirus group, contains a circular DNA genome, partially double helix, has a molecular weight of 3.2 kb, including 4 genes: C, P, S and X.
Currently, We currently have three classes of oral HBV antiviral drugs that are commonly used to treat chronic hepatitis B:
L-Nucleoside group includes: Lamivudine (LMV), Emtricitabine (FTC), Telbivudine (LdT) ) and Clevudine (L-FMAU). The Acyclic Phosphonate group includes: Adefovir dipivoxil (ADV) and Tenofovir (ADV). Cyclopantane/Pentene cyclic group includes: Entecavir (ETV), Abacavir/ Cabovir (ABC). These drugs work by inhibiting the reverse polymerase-enzyme that catalyzes reverse transcriptase from mRNA to DNA of the HBV virus. Therefore, they reduce the replication of HBV virus, thereby reducing the rate of cirrhosis complications, hepatocellular carcinoma and the risk of patient death.
However, long-term treatment with these drugs can lead to the emergence of drug-resistant mutations in the P gene of the HBV virus, making the drug ineffective, leading to treatment failure and making the treatment worse. Hepatitis continues to progress.
To date, many types of HBV resistance mutations have been discovered by scientists and all these mutations are located on the reverse transcriptase region of the P gene. Gene P is divided into 7 domains (domains) . of the enzyme reverse polymerase (rt). Changes in amino acids in common resistance mutations include:
rtL80I/V mutations in the A region. rtI169T, rtV173L, rtL180M, rtA181T/V/S, rtT184A/S/G/C mutations in the A region. B. The rtA194T, rtS202C /G/I, rtM204V/I mutations in the C region. The rtN236T mutations in the D region. The rtM250V mutations in the E.
Cấu trúc của virus viêm gan B (HBV) và genome của nó
These amino acid changes can occur individually or in combination, conferring varying degrees of resistance to these drug-resistant strains of HBV.
The resistance mutations of HBV during single drug treatment are:
Lamivudine resistance mutations include: rtL180M + rtM204V/I/S, rtM204I, rt80VI + rtM204I, rtV173L + rtL180M mutations + rtM204V, rtI169T + rtV173L + rtL180M + rtM204V/, rtA181T, rtT184S + rtL180M + rtM204V or rtQ215S + rtL180M + rtM204V. Adefovir resistance mutations include: rtN236T, rtA181V/T, rtV84M/ rtS85A/ rtL80V/I or rtV214A/ rtQ215S mutations. Entercavir resistance mutations include: rtI169T + ntV173L + rtL180M + rtT184G + rtM204V, rtI169T + ntV173L + rtL180M + rtM204V + rtM250V, or a combination of mutations occurring at codons 184, 202 and 250. Telbivudine resistance includes: rtM204I mutations or Lamivudine resistance-related mutations. Tenofovir resistance mutations include: rtL180M + rtA194T + rtM204V, rtV204A, rtQ215S or rtA181V + rtM204I mutations.
HBV multidrug resistance mutations when combined with two or more drugs include:
Lamivudine and Adefovir resistance mutations include:
rtM204I + rtL180M rtM204V + rtL180M rtM204I + rtM204V + rtL180M Mutations Lamivudine and Entecavir resistance include:
rtM204V + rtL189M + rtT184L rtM204V + rtL189M + rtS202G rtM204V + rtS202G. Lamivudine, Adefovir and Entecavir resistance mutations include:
rtA181T + rtM204V + rtS202G rtA181T + rtM204V + rtL180M + rtT184L. Lamivudine and Tenofovir resistance mutations include:
rtV214A, rtQ215S, ±rtL180M + rtM204V rtL180M + rtA194T + rtM204V Resistance mutations to Lamivudine, Adefovir and Tenofovir include: rtA184I + rtM181V mutations.

Điều trị HBV kháng thuốc cần kết hợp một số loại thuốc với nhau
2. How to identify HBV drug resistance mutations
How hepatitis B virus (HBV) resistance mutations can be identified: Mutations located at certain positions in the reverse transcriptase domain (rt region) of the HBV polymerase gene have been shown to be present. role in the development of drug resistance in HBV.
To identify HBV drug-resistant mutations, doctors will use nested PCR technique to multiply the rt region with primer pairs as follows:
Outer PCR includes HBV-69 and HBV-70. Inner PCR primers include HBV-61 and HBV-17. The inner ring PCR product is then purified for sequencing. The sequence of the target gene segment after sequencing will be compared with the reference sequences of the wild-type strains and the reference sequences with different genotypes to compare and search for resistance mutations based on a software called Bioedit. In the course of treatment, the identification of drug resistance mutations of HBV in patients with chronic hepatitis who manifest drug resistance is essential for the doctor to decide whether to change a new drug or use a combination of drugs. appropriate way to achieve the most effective treatment.
Ideally, before using medicine to treat hepatitis B, patients should discuss with their doctor about their current health status in order to have the most appropriate indications.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.