This is an automatically translated article.
Posted by Resident Doctor, Doctor Nguyen Thi Thuy Hang - Doctor of Microbiology - Laboratory Department - Vinmec Times City International Hospital
The HBcrAg biomarker can provide clinicians with reliable information about the replication activity of the hepatitis B virus in HBV-infected patients in a noninvasive manner.
1. Make a problem
Patients with HBV are typically monitored with biomarkers such as HBV DNA, HBsAg (quantitative) and ALT. However, it is difficult for clinicians to immediately identify patients who require treatment or establish reliable discontinuation rules. cccDNA acts as a template for viral replication and is responsible for viral persistence in the liver even after HBsAg loss and seroconversion.Measurement of HBV cccDNA can be important for clinical decision making. However, standardized tests are needed and current cccDNA measurement methods require liver biopsy.
In March 2017, the European Association for the Study of the Liver (EASL) added a new serological marker, HBcrAg (hepatitis B virus core-associated antigen), to the list of markers. Promising new biology of HBV.
2. What is HBcrAg?
What is HBcrAg? HBcrAg consists of three proteins encoded by the precore and core region genes, including the hepatitis B virus envelope antigen (HBeAg), the hepatitis B virus core antigen (HBcAg), which is the main component of the hepatitis B virus. The Dane particle (Dane particle) and the 22 kDa precore protein (22-kDa precore protein: p22cr) is an HBV DNA-negative empty particle. By serological testing, the measured serum HBcrAg level is the sum of the markers HBeAg, HBcAg and p22cr.
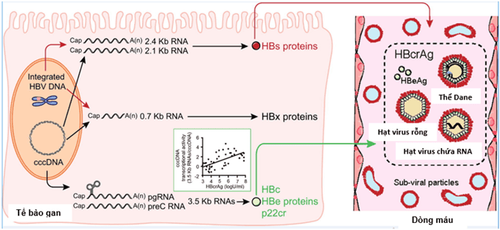
3. Value of HBcrAg . marker
3.1. In the natural course of disease, HBcrAg biomarkers can provide clinicians with reliable information about the replication activity of the hepatitis B virus in HBV-infected patients in a noninvasive, noninvasive manner. liver biopsy, instead of cccDNA quantification. Studies have shown that serum HBcrAg levels reflect cccDNA levels in hepatocytes, both in HBeAg-positive and HBeAg-negative patients.
Serum HBcrAg levels vary significantly between different stages of HBV infection. Therefore, HBcrAg is also a good marker to distinguish HBeAg-negative chronic hepatitis B patients (active disease) from HBeAg-negative chronic HBV infection (inactive disease).
HBcrAg helps predict HBeAg seroconversion. Currently, in clinical practice, qualitative HBeAg does not provide this value; while some institutes have implemented quantitative HBeAg to monitor HBeAg seroconversion. However, the quantitative HBeAg test is actually a calculation formula from the qualitative HBeAg, so the results have not been evaluated in scientific studies. Meanwhile, HBcrAg as a novel test marker has a high value in predicting HBeAg seroconversion and the optimal cut-off of HBcrAg has also been demonstrated in scientific studies.
3.2. In the course of antiretroviral drug therapy Currently, the Ministry of Health has issued guidelines for using HBcrAg in combination with HBeAg, HBsAg and HBV-DNA to measure the load to identify patients who can stop nucleotide(s) therapy. )ide analogue (NA).
3.3. In HCC progression, HBcrAg may be a tool to assist clinicians in identifying patients at higher risk of developing HCC during nucleotide(s)ide analog therapy. NA) or post-treatment recurrence of hepatocellular carcinoma (HCC).
In summary, HBcrAg is a non-invasive marker and has great potential to become a useful tool to replace cccDNA for disease monitoring, predicting response to treatment of chronic hepatitis B virus.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.