This is an automatically translated article.
Posted by Master, Doctor Mai Vien Phuong - Department of Examination & Internal Medicine - Vinmec Central Park International General Hospital
Many new endoscopic techniques have been shown to increase the likelihood of early gastric cancer diagnosis. With improved early gastric cancer detection rates, endoscopic treatment has gained popularity due to advances in existing equipment and the experience of endoscopists. The purpose of this review is to summarize the endoscopic diagnosis and treatment commonly used in early gastric cancer (early gastric cancer).
1. Introduction to early gastric cancer
Stomach cancer is the fourth most common cancer worldwide and the second leading cause of cancer death. With increasing public awareness of early cancer diagnosis and treatment as well as the development of endoscopic imaging and imaging enhancement techniques, such as magnified narrowband imaging, endoscopic staining color and confocal laser endoscopy, the rate of early gastric cancer at diagnosis is increasing. Early detection is essential for treatment. It has been shown that the prognosis of early gastric cancer is excellent with 5-year survival rates above 90%.
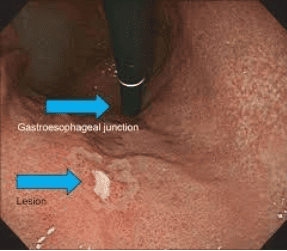
2. The role of diagnostic endoscopy by white light endoscopy
The world's guideline "Consensus on diagnosis, treatment and endoscopic screening for early gastric cancer, 2014" recommends high-risk populations: (1) > 40 years of age; (2) Helicobacter pylori infection; (3) previous precancerous diseases such as chronic atrophic gastritis, gastric polyps, peptic ulcer, pernicious anemia; and (4) other high risk factors such as alcohol, smoking, high salt, preserved foods.
White light endoscopy (conventional white light endoscopy) can only detect obvious morphological changes of cancerous lesions, such as changes in their color (red), surface contour , or the kinetic response to gas injection/suction. Early gastric cancer can be divided into 3 types: raised, superficial and concave. Shallow type is subdivided into raised appearance, flat appearance and concave appearance. Superficial flat lesions are difficult to find during routine white light endoscopy, often causing misdiagnosis and missed diagnoses. The most common lesions of early gastric cancer usually present with congestion and ulceration.
2.1 Magnified endoscopy with NBI . narrowband imaging
Magnified endoscopy with narrow band imaging (Enlarged endoscopy with narrow band imaging) is a recently developed technique that is a combination of magnified endoscopy and narrowband imaging. It is widely used in early gastric cancer detection based on the basic microbiological findings of the microvascular structure (microvascular) and microstructure (microsurface) of the superficial mucosa. outside. The gastric mucosa is composed of glandular epithelium, which is different from normal glands. There are different classification systems to describe the correlation between microscopic images and actual images visualized by Magnified Endoscopy with narrowband imaging.
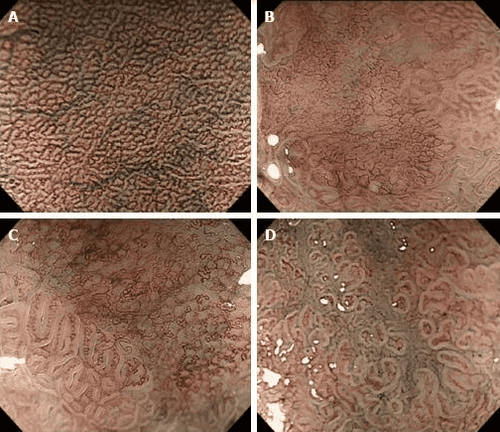
Microvascular and microsurface patterns reported by Yao et al where three microvascular/surface patterns are described: regular, irregular, and absent. According to these microsurface/microvascular patterns, one can distinguish low-grade dysplastic gastric adenomas from early gastric cancer or determine the extent of lateral spread and depth of invasion of the tumor. of early gastric cancer for laparoscopic resection. The diagnostic criteria for gastric cancer depend on the presence of an irregular microvascular/microsurface pattern with a borderline. It is worth mentioning that 97% of early gastric cancers fit the above criteria.
2.2 Confocal Laser Magnification Endoscopy
Confocal laser magnifying endoscopy (CLE) is a newly developed endoscopic imaging technology that produces 1000x magnification cross-sectional images of the gastrointestinal tract surface and subsurface tissue . It is capable of providing direct histological observation of tissue in vivo without biopsy and distinguishing malignant from benign lesions in real time at the cellular level.
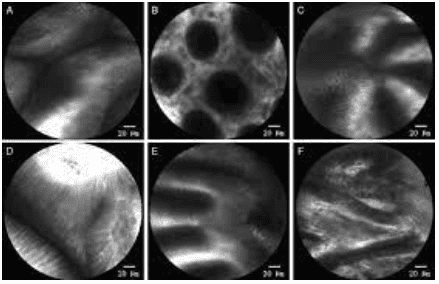
3. Endoscopic treatments
New NCCN guidelines recommend that endoscopic mucosal resection or endoscopic submucosal dissection of early gastric cancer may be considered appropriate therapy when lesions with a diameter of ≤2 cm; It is shown histopathologically that it is well or moderately differentiated, does not penetrate beyond the superficial submucosa, exhibits no lymphatic invasion, and has clearly lateral and deep margins. But the guidelines do not specify when endoscopic mucosal resection is and when endoscopic submucosal dissection is indicated.
The following are the risk factors in the case of endoscopic therapy, where endoscopic resection may not be possible: (1) the lesion cannot be raised after submucosal saline injection (signs of without lifting), (2) early gastric cancer with lymph node metastasis, (3) cancer that invades the muscularis stroma, and (4) severe coagulation dysfunction. Age is not a risk factor, except in cases of severe organ failure. Anticoagulation should be discontinued 5–7 days prior to the procedure.
3.1 Endoscopic mucosal resection EMR
Endoscopic mucosal resection was first applied to endoscopic therapy in 1984 by means of a strip biopsy (two-channel method). The procedure involves submucosal injection below the lesion, excision, and excision of the lesion. This lifting injection method is very simple and convenient. However, it is difficult to trap flat lesions. Furthermore, metal noses are prone to slippage, which can lead to incomplete excision and local recurrence.
3.2 The technique of creating an annular section before mucosal excision EMR-P
Endoscopic mucosal resection after circumcision was described by Hirao et al in 1988. The injection-precutting-snaring technique. -P) refers to submucosal injection of hypertonic saline mixed with diluted epinephrine, cutting around the lesion with a needle knife, and excision of the lesion with a specialized noose. In summary, the technique of mucosal resection after injection and the technique of creating an annular section before cutting the mucosal cavity are methods that do not aspirate the lesion into the procedure channel. Aspiration techniques for endoscopic mucosal resection include endoscopic mucosal resection with CAP (endoscopic mucosal resection -C) and endoscopic mucosal resection. endoscopic mucosal ligation (endoscopic mucosal resection - L). Traditional endoscopic mucosal resection procedures (mentioned above) cannot remove flat lesions larger than 2cm at a time. This type of lesion can be removed by endoscopic partial mucosal resection (EPMR). However, it is difficult to graft resected samples in vitro and evaluate the effect of radical resection after EPMR.
3.3 Endoscopic submucosal dissection (ESD) technique
Given the limitations of endoscopic mucosal resection, new endoscopic techniques are sought to remove larger tissues. Endoscopic submucosal dissection (endoscopic submucosal dissection) allows for mass resection of larger lesions. The steps of this endoscopic technique include marking, submucosal injection, circumferential mucosal resection, dissection, and wound management. Current indications for endoscopic submucosal dissection are based on the criteria reported by Gotoda et al, including early gastric cancerous mucosal ulcers <3 cm diameter or depth Submucosal invasion of early gastric cancer ≤3 cm.3.4 Endoscopic submucosal tunneling technique
Endoscopic submucosal tunneling (ESTD) was first introduced by Linghu et al as a novel strategy for rapid resection of large esophageal tumors. A tunnel is established between the mucosa and the muscularis stroma; After that, the lesions are healed quickly. The use of endoscopic submucosal tunneling to remove early gastric cancer has some limited indications such as early gastric cancer with severe fibrosis due to previous endoscopic submucosal dissection. or severe ulceration and adequate resection is achieved due to submucosal invasion. Choi et al. reported two cases of early gastric cancer with ulcerative submucosal fibrosis that were treated by endoscopic submucosal dissection. There is no denying that endoscopic submucosal tunneling has several advantages. For example, bleeding is easier to control because blood vessels are clearly exposed, and surgery time is shortened. More studies are needed to evaluate the safety and efficacy of endoscopic submucosal tunneling for early resection of gastric cancer.
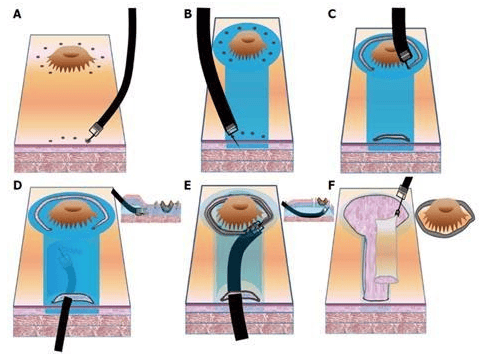
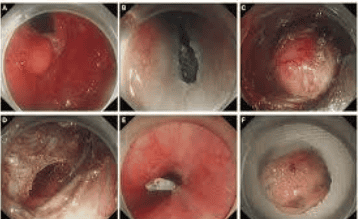
In summary, with the development of endoscopic techniques, more prospective studies with high quality design are needed to evaluate the diagnostic accuracy of these new endoscopic imaging techniques to complemented treatment outcomes, survival, complication rates, and procedure standardization, and developed a learning system widely accepted by future endoscopists.
Vinmec International General Hospital is equipped with the most modern flexible endoscopic system of Olympus - Japan. Narrow Banding Imaging (NBI) has made a breakthrough in the screening and diagnosis of cancers of the gastrointestinal tract (esophagus, stomach, duodenum, colon, rectum). in the early and very early stages. NBI endoscopic images have high resolution and contrast, so it is easy to detect small changes in color, morphology of cancerous and precancerous lesions that are difficult to detect with conventional endoscopy.
Standard treatment techniques, ensuring safety and quality of treatment for patients. The combination of a team of qualified and experienced specialists with a modern diagnostic endoscopy system, including the NBI narrow-band endoscopy system, you will enjoy the complete quality of service. Good quality and good diagnostic and treatment quality for gastrointestinal tract diseases.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References1. Jemal A., Bray F., Center M. M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer Journal for Clinicians . 2011;61(2):69–90. doi: 10.3322/caac.20107. [PubMed] [CrossRef] [Google Scholar]
2. Min Y. W., Min B.-H., Lee J. H., Kim J. J. Endoscopic treatment for early gastric cancer. World Journal of Gastroenterology. 2014;20(16):4566–4573. doi: 10.3748/wjg.v20.i16.4566. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
3. Oliveira F. J., Furtado H., Furtado E., Batista H., Conceicao L. Early gastric cancer: report of 58 cases. Gastric Cancer . 1998;1(1):51–56. doi: 10.1007/s101200050054. [PubMed] [CrossRef] [Google Scholar]
4. Ono H., Kondo H., Gotoda T., et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut . 2001;48(2):225–229. doi: 10.1136/gut.48.2.225. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
5. Linlin Zhu, 1 Jinyu Qin,, Early Gastric Cancer: Current Advances of Endoscopic Diagnosis and Treatment, Gastroenterol Res Pract. 2016; 2016: 9638041.