This is an automatically translated article.
Posted by Doctor, Specialist II Le Thi Na - Doctor of Hematology - Laboratory Department - Vinmec Times City International Hospital
Iron is a trace element that plays an important role in the body, synthesizing hemoglobin. Iron is required for the functioning of many proteins and is involved in redox reactions, controls energy production, mitochondrial respiration, and DNA synthesis. The total amount of iron in the body is equal to 0.008% of body weight.
1. Iron distribution in the human body
| Khu vực | Nam (mg Fe/kg) | Nữ (mg Fe/kg) |
| Chức năng | ||
| Hemoglobin | 31 | 28 |
| Myoglobin | 5 | 4 |
| Các enzyme | 2 | 2 |
| Dự trữ | ||
| Ferritin và hemosiderin | 12 | 6 |
| Vận chuyển | ||
| Transferrin | < 1 (0,2) | < 1 (0,2) |
| Tổng cộng | 50 | 40 |
In the body, iron is distributed into 3 areas: function, transport and storage.
Functional Areas: About two-thirds of the iron in the body is in the functional area, mainly in hemoglobin. One gram of hemoglobin contains 3.3 mg of iron, one milliliter of red blood cell concentrate contains one mg of iron. The amount of iron in myoglobin is very low but is present in all skeletal muscle and heart cells. A very small amount of functional iron (6-8 mg) is present in cytochromes and enzymes, especially in ribonucleotide reductase. Therefore, iron plays a role in the metabolism of all cells.
MORE: Pregnancy ferritin test
Transport area : Iron in the transport area makes up about 0.1% of the body's iron. In plasma, iron is transported as Fe3+ bound to transferrin. The role of transferrin is to dissolve and bind Fe3+ in physiological form (avoid leaving iron in free form) and to transport iron supplies to cells through iron-binding receptors.
Storage area: About 30% of the body's iron is in the form of stores, of which 60% is in the liver and 40% in the reticuloendothelial system. In the liver, over 95% of iron is as ferritin in hepatocytes, the rest in Kupffer cells as hemosiderin. In the spleen and bone marrow, iron is mainly stored in the interretinal endothelial cells. Iron makes up 20-25% of the ferritin molecular weight. Ferritin is a source of iron for the synthesis of hemoglobin in red blood cells. As red blood cells increase the need for hemoglobin synthesis, the amount of intracellular iron as well as the amount of iron in the ferritin molecule decreases.
Free ferritin in serum reflects iron stores. Ferritin levels are elevated in cases of iron overload. In addition, elevated ferritin is also seen in cases of tumors, acute and chronic inflammation. Hemosiderin is an iron-protein complex, made from ferritin, about 10% of ferritin has a tendency to form hemosiderin, visible hemosiderin under light microscope after staining with ferrocyanure de potassium (Perls). Hemosiderin is a semi-crystalline concentrate of ferritin concentrated mainly in the liver, spleen, and bone marrow. In cases of iron overload, the amount of hemosiderin can be accumulated up to 10 times higher than ferritin. The iron in hemosiderin is more difficult to release than the iron in ferritin.
MORE: What causes an increase in Ferritin?
Chuyển hóa sắt trong cơ thể có liên quan đến hồng cầu
2. Iron metabolism
There are 3 main factors affecting iron balance and metabolism: the process of receiving, storing and losing. Most iron metabolism is carried out in a closed system between sites. In adults, 95% of the iron required for erythropoiesis is recycled from the breakdown of aged red blood cells, with only 5% of the additional iron obtained from food. The body only needs to provide 1 mg of iron/day to meet the needs of normal red blood cells. The process of digestion and absorption of iron begins in the stomach but mainly in the duodenal bulb and the first part of the jejunum. The control of iron absorption and the amount of iron transported into the portal blood depends on the body's iron requirements and iron stores. In case of iron overload in the body, the amount of iron absorbed into intestinal epithelial cells will decrease.
Iron transport and utilization: During the physiological aging of red blood cells, the cell membrane structure is changed, it is easy to bind to IgGs, which is a signal for macrophages in the liver and spleen to reach the food. cell. Every day, about 1/120 of the number of red blood cells are phagocytosed, producing about 16.5 - 20 mg of iron, this amount of iron is released into the blood, transported by transferrin to the bone marrow to create new red blood cells. When the transferrin - Fe3+ complex enters the cell, Fe3+ binds to the transferrin receptor on the surface of the target cell and is transported into the cell. HFE is a protein involved in the regulation of iron metabolism, HFE competes with transferrin - Fe3+ at the TfR1 site. The HFE - TfR1 complex prevents TfR1 from binding to transferrin - Fe3 +, so iron will not be able to be transported into the cell. However, HFE does not bind to TfR2, so TfR2 can transport iron unlimitedly into cells, causing excess iron in cells such as liver cells, heart cells, and endocrine glands. In the cell, iron is transported to the mitochondria, where it is attached to protoporphyrin for heme synthesis or stored in ferritin. Ferroportin is a transmembrane iron transport protein, ferroportin is present in gastrointestinal epithelial cells, hepatocytes, kupffer cells and macrophages. In the body, the hormone hepcidin regulates the action of ferroportin.
Regulation of iron metabolism in cells: The most important substance in the regulation of iron metabolism is hepcidin, which is produced by the liver. Hepcidin regulates iron absorption by intestinal mucosal cells and regulates iron release by macrophages. Hepcidin inhibits 23 ferroportin by binding and breaking down ferroportin, leading to decreased absorption of iron from food into intestinal epithelial cells. Hepcidin inhibits iron release from macrophages. In normal people, when the body lacks iron, the liver reduces the synthesis of hepcidin. Then ferroportin is released, transporting iron from the food into the intestinal epithelium, and then into the portal vein system. Hepcidin decreased, macrophages increased release of iron stored in ferritin. When the body has an excess of iron, the liver increases the synthesis of hepcidin, leading to a decrease in ferroportin. Ferroportin decreases, causing intestinal epithelial cells to reduce iron absorption. Hepcidin increases, macrophages limit the release of stored iron in ferritin.
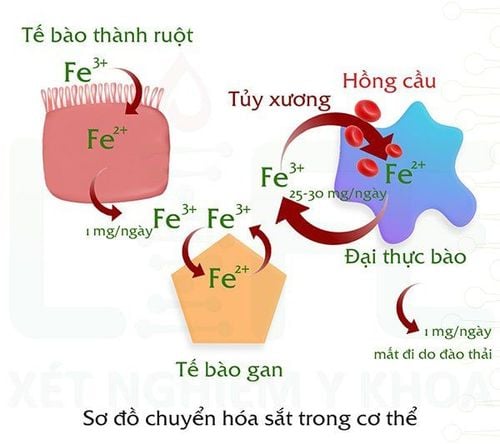
Sơ đồ chuyển hóa sắt trong cơ thể
3. Application
The amount of iron provided daily in a normal diet meets the body's iron needs. In the case of increased iron requirements due to iron loss (blood loss) or increased iron requirements (pregnant women, young children, puberty) it is necessary to supplement iron in the diet and supplement iron products. When there are manifestations of anemia or manifestations related to iron excess, it is necessary to examine the cause to see if it is related to iron metabolism or not to take appropriate corrective measures.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.