This is an automatically translated article.
Post by Master, Doctor Phan Truc - Internal Medicine Oncologist - Hematology - Oncology Center - Radiation Therapy - Vinmec Times City International Hospital
In acute lymphoblastic leukemia, marrow examination is recommended for the diagnosis of this disease because approximately 10% of patients do not have circulating blasts at the time of diagnosis and the high density of myeloid cells favors the diagnosis. gene research.
1. Cell diagnosis and classification
Bone marrow examination is recommended for the diagnosis of ALL, because approximately 10% of patients do not have circulating blasts at the time of diagnosis and because the high density of myeloid cells favors genetic studies. Pulp that is fibrous or too dense can be difficult for aspiration, in which case a pulp biopsy should be performed. In patients with bone marrow necrosis, multiple punctures may be needed to obtain appropriate tissue samples.
1.1. Morphological and cytochemical analysis The diagnosis of ALL begins with analysis of the Romanowsky staining (Wright-Giemsa or May-Grunwald-Giemsa) smears of the marrow smear. Lymphoblasts tend to be relatively small (ranging from up to twice the size of small lymphocytes) with a narrow cytoplasm, often pale green; round or slightly concave kernel; fine to slightly coarse, clustered and discreet nuclei. In some cases, large lymphoblasts, prominent nuclei, moderate cytoplasmic content, mixed with smaller blasts. Cytoplasmic granules have been observed in some patients with ALL. The seed is fuchsia, easily distinguishable from the myeloid-specific seed (dark purple), and shows it to be mitochondrial under the electron microscope. The B-cell lineage blast cells in Burkitt-type ALL are characterized by strongly basophilic cytoplasm, prominent nuclei, and numerous cytoplasmic vacuoles. Cytochemical staining (Sudan Black, Myeloperoxidase, Nonspecific Esterase) helps distinguish ALL from AML, but is now less common because immunophenotypic analysis is available.
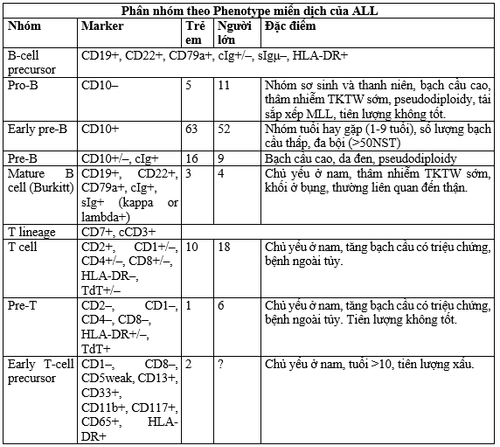
1.2. Immunophenotyping is an essential part of the diagnosis. Antibodies help differentiate CD groups (cluster of differentiation), but most leukocyte antigens are nonspecific. Therefore, an antibody panel is required for the diagnosis and classification of the subtype of leukemic cells. The classic panel includes antibodies for at least one highly sensitive marker (CD19 for B cells, CD7 for T lineage, CD13 or CD33 for myeloid) and antibodies for highly specific markers (cytoplasmic CD79a and CD22 for the B lineage, CD3 in the cytoplasm for the T line, and cytoplasmic myeloperoxidase for the myeloid line). Although ALL can be further classified according to each maturity step in each line, the B line (pro-B, early pre-B, pre-B, transitional pre-B and mature B), the T line (pre-T) , mid- and late thymocyte), the most important classification currently relevant to treatment is between T lineage, adult B lineage and other B-cell precursors. Another subgroup of the T-lineage that has the appearance of stem cells is early T-cell precursor ALL, which has a very poor prognosis with current chemotherapy. Knowing the antigenic components that are present at the time of diagnosis is paramount in determining minimum residuals (MRD) by post-treatment flow cytometry.
Myeloid-associated antigens may be abnormally expressed in lymphoblasts. The frequency of expression of these myeloid antigens is seen in 5-30% of children and 10-15% of adults. The myeloid antigenic component expressed is related to blast cell genetics. CD15, CD33, CD65 expressed in ALL with MLL gene rearrangement; CD13 and CD33 were expressed in cases with ETV6-RUNX1. There was one subgroup that simultaneously expressed lymphocytic and myeloid markers that could not be classified. This case did not respond to myeloid chemotherapy, but did respond to ALL induction therapy. The presence of myeloid antigens has no prognostic significance in current treatment programs but may be useful in monitoring MRD.
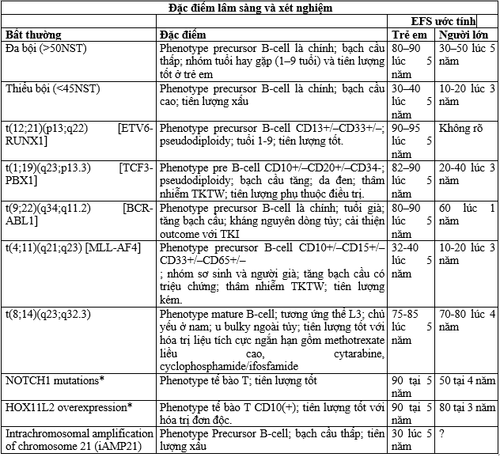
1.3. The genetic (gene) classification of ALL derives from a lymphoid progenitor cell that has undergone many specific genetic alterations leading to malignant transformation and proliferation. Therefore, gene classification in ALL is more biologically accurate than any other classifier. Nearly 75% of cases in children and adults have been classified into treatment- or prognostic-related groups based on chromosome number (or estimation of DNA volume by flow cytometry), specific chromosomal rearrangement, and alteration. inherited at the molecular level. The two groups with changes in the number of chromosomes (polyploidy > 50 chromosomes, aneuploidy <44 chromosomes) are clinically related. Polyploidy, seen in 25% of children, 6-7% of adults, is associated with a favorable prognosis because it increases intracellular accumulation of methotrexate and its polyglutamate resulting in increased sensitivity to anticonverting agents. and these cells undergo apoptosis. In contrast, hypoplasia is associated with a significantly poor prognosis. Determination of DNA by flow cytometry is useful in cytogenetic analysis because it is automatic, fast and cheap, and is not affected by the mitotic index of the cell population; results can be obtained in most cases. Flow cytometry studies can also identify a small number of drug-resistant near-haloid populations that would be missed by standard genetic analyses.
Reciprocal chromosomal translocation in cases of B-ALL, T-ALL comes from errors in the normal recombination process, creating antigen receptor genes. Such rearrangements may fuse the promoter/enhancer portion of the heavy- or light-chain Ig gene, or the T-cell antigen receptor gene, to the site of the transcription factor (TF) gene. More commonly, the rearrangement produces a combination of two genes coding for a different transcription factor. These genes encode active kinases and altered transcription factors that regulate differentiation, self-renewal, proliferation, and drug resistance in hematopoietic stem cells.
Discoveries in cytogenetic analysis are closely related to clinical features, blast cell phenotype, and clinical outcome. However, there are compelling reasons to focus on genetic damage at the molecular level. First, molecular analysis can identify important submicroscopic genetic changes that cannot be observed with standard karyotypes such as the ETV6-RUNX1 combination, chromosome 21 amplification, and suppressor gene deletions. tumor suppressor, and protooncogene mutations. Second, many cases of clinically important genetic rearrangements can be missed by technical error.
2. Differential diagnosis
The initial manifestations of ALL can resemble a variety of disorders. The acute onset of petechiae, ecchymosis, and bleeding may suggest idiopathic thrombocytopenic purpura. The latter is usually associated with recent viral infection, large platelets in the blood smear, normal hemoglobin levels, and no abnormal white blood cells in the blood or marrow. Patients with ALL, or promyelocytic leukemia, or aplastic anemia may present with 3-line cytopenias and complications related to myelosuppression. However, in aplastic anemia, hepatosplenomegaly and lymphadenopathy are rare and bone pain associated with ALL is absent. The results of aspiration or biopsy often distinguish between these diseases, although the diagnosis can be difficult in a patient whose poor marrow density is replaced by lymphoblasts. In one study, a 3-line transient reduction precedes ALL in 2 percent of pediatric patients. In the pre-leukemic stage in these patients, polymerase chain reaction (PCR) analysis demonstrated monoclonal, suggesting suppression of normal hematopoiesis of leukemic cells. ALL should be considered in the differential diagnosis of patients with eosinophilia, which may be a feature of leukemia or may precede its diagnosis by several months. Occasionally, regenerative marrow hematogone can mimic leukemic blast cells; flow cytometry with an optimal combination of antibodies may be required to differentiate them.
Infectious mononucleosis and other viral infections, especially those associated with thrombocytopenia or hemolytic anemia, can be confused with leukemia. Detection of reactive lymphocytes or serological evidence of Epstein-Barr virus infection helps establish the diagnosis. The patient also developed lymphocytosis following acute infection or pertussis. However, even when the white blood cell count is as high as 50 × 109/L, the affected cells are mature lymphocytes rather than lymphoblasts. Bone pain, joint pain, and sometimes arthritis mimicking juvenile rheumatoid arthritis, rheumatic fever, other collagen diseases, or osteomyelitis. A marrow examination is required if glucocorticoid therapy is planned for rheumatic diseases.
In children, ALL should be differentiated from small, round cell tumors involving the marrow, including neuroblastoma, rhabdomyosarcoma, and retinoblastoma. Usually, in patients with solid tumors, a primary lesion will be found by standard diagnostic studies. Common tumor cells are often present in characteristic aggregates, and immunological features of lymphoblasts are absent.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.