This is an automatically translated article.
The article was written by Dr. Pham Thi Viet Huong - Doctor of Hematology - Oncology - Hematology and Cell Therapy Unit - Vinmec Times City International General Hospital.Chylous pleural effusion is a rare complication of both traumatic and non-traumatic events, occurring in approximately 2% of adult pleural effusions. An estimated 2.4 liters of chyme is transported each day through the lymphatic system. Injury or rupture of the thoracic duct can cause a rapid and massive accumulation of chyme in the pleural space.
1. Chylous pleural effusion in a clinical case of large B-cell non-Hodgkin lymphoma
First, increased pressure in the thoracic duct can cause retrograde chyme outflow through the parietal pleura lymph into the pleural space. Another mechanism is the invasion of lymphoma into the thoracic duct, which makes the thoracic duct susceptible to rupture. Lymph drains into the pleural space, causing nutritional, metabolic, immunological and respiratory complications. The most common non-traumatic cause is malignancy, especially lymphoma. Chylous pleural effusion is a rare complication of both Hodgkin lymphoma and non-Hodgkin lymphoma (ULAKH). The pleural effusion in lymphoma is different from the pleural effusion due to other cancers and has both transudative and exudative features. This condition can be dangerous and fatal, with an estimated 10% of mortality and morbidity on average. Chylous pleural effusions are uncommon and are rarely described in ULAKH of any histology or malignancy. Although patients often have diffuse lymphoma, lesions on the diaphragm are not always present. This serious clinical complication may not be apparent at the time of diagnosis. It is usually a chronic complication, and its course does not reflect successful treatment of the lymphoma. Confirm the diagnosis by quantifying cholesterol and triglycerides in the pleural fluid. Treatment can be either conservative or aggressive depending on the clinical setting.Treatment of chylous pleural effusion is often long and depends on the type, its duration, the degree of chylous loss and the general health of the patient. In general, the important solutions to the treatment of chylous pleural effusion include effective cancer therapy and complementary interventions such as parenteral nutrition, treatment of concomitant infections as well as rehabilitation. power. Recurrent cancer-associated chylous effusions are generally managed pleurally with talcum powder or by indwelling pleural catheter (IPC) in a palliative care setting to reduce complications. symptoms and improve quality of life. We report a clinical case of persistent, persistent chylous effusion in a patient with ULAKH who had long-term remission and complete resolution of the chylous pleural effusion after combined chemotherapy and emergency treatment. by placing drainage of the membrane. The objective of this paper is to present our experience in the conservative management of malignant non-traumatic chylous pleural effusion.
Tràn dịch dưỡng trấp màng phổi là biến chứng hiếm gặp và cần điều trị lâu dài
2. Description of the clinical case
A 72-year-old female patient was diagnosed with non-Hodgkin lymphoma, 4 weeks before admission, the patient appeared fatigue, anorexia, acute shortness of breath, or night sweats. In addition, the patient presented with a feeling of heaviness in the head and transient facial swelling. The patient before being admitted to the Emergency Department (ICU) of Vinmec Times City Hospital at 10 pm on August 7, 2020. At the time of admission, the patient felt increased shortness of breath, swelling of the legs and face, and oliguria. The patient had a 10-year history of hypertension and a 6-year history of stroke. No history of pre-existing lung or autoimmune disease, recent trauma or surgery, no smoking.At the Intensive Care Unit from August 7, 2020 to August 12, 2020, the patient was very tired and weak; Blood pressure 140/79 mmHg, heart rate 79 beats/min, respiratory rate 28 beats/min and SpO2 92% when breathing air, ECOG is 3. Clinical examination decreased ventilation, the patient's alveolar murmur gradually decreased in Right chest with low-grade percussion. The patient had generalized edema and had a firm, non-motile left supraclavicular node, measuring 3 cm. Abdominal examination revealed a nodular mass occupying the hypogastrium and umbilicus. Chest X-ray showed a large right pleural effusion. Whole-body computed tomography revealed a tumor of the superior mediastinum and mediastinum measuring 102 x 104 mm, numerous cervical, axillary, aorta and mesenteric lymph nodes with the largest size of 190 x 120 mm and surrounds the abdominal aorta and the iliac arteries. Full-body computed tomography also showed pelvic dilatation, 46 mm of free intra-abdominal fluid, bilateral pleural effusion and pericardial effusion, and no hepatosplenomegaly. The patient's dyspnea improved significantly after thoracoscopy, which aspirated 500ml of milky white fluid from the right pleural space daily (Figure 1). Pleural fluid is usually an exudate with a triglyceride level of 5.25 mmol/L, lipase 19.8 U/L and cholesterol 3.13 mmol/L. The number of white blood cells in the pleural fluid is 5018 cells/mm3, lymphocytes 20%, neutrophils 80%. Pleural fluid has a protein content of 41.7 g/l and pleural fluid LDH is 368 IU/L. The pleural fluid analysis was negative for malignant cells. Pleural fluid culture was negative. Diagnosis of chylous pleural effusion was confirmed by lipoprotein electrophoresis of the pleural fluid, demonstrating the presence of chylomicrons; Triglyceride in pleural fluid is 5.25 mmol/L. Our patient has acute renal failure. Echocardiography showed atrial fibrillation with a heart rate of 134-170 beats/min, arrhythmias, normal cardiac function with an ejection fraction of 67%, and pericardial effusion. Bone marrow aspiration could not be performed because the patient had difficulty breathing. Real-time PCR MTB/NTM test showed that the patient was not infected with mycobacterium tuberculosis, not infected with non-tuberculosis mycobacteria.

Hình 1: Dịch dưỡng trấp dẫn lưu từ khoang màng phổi phải của bệnh nhân
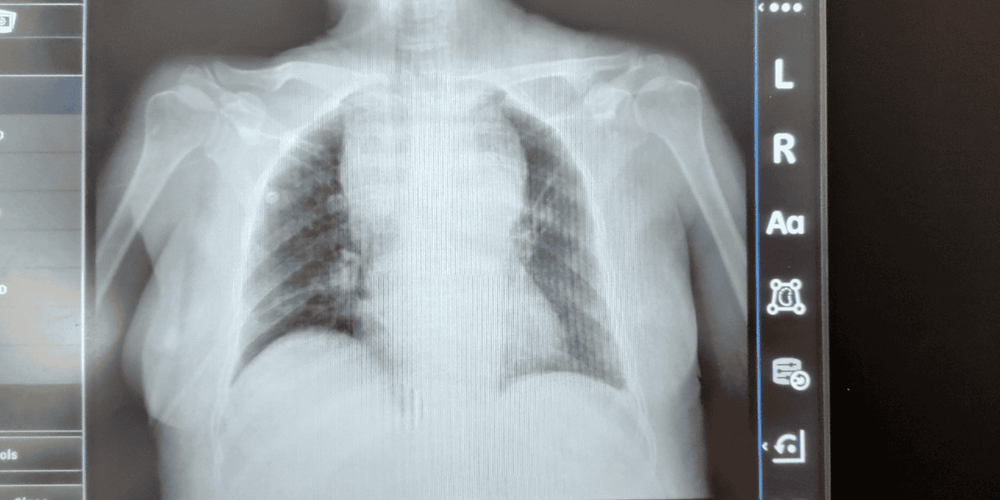
Hình 2: Chụp X quang tại giường ngày 13/8/2020 sau dẫn lưu dịch màng phổi
The patient was transferred to our Inpatient Department of Stem Cell Transplant Hematology Unit after 5 days of care in the ICU. Biopsy of the left supraclavicular lymph node suggested diffuse large B-cell ULAKH with CD 20+ germline type. Immunohistochemical staining results: CD20+; CD10+; BCl6+; MUM1-; CD79a+; PD1-; ALK-; CD30-; CD3- ; CD5-; CD4-; CD8-; TdT. The patient was classified as stage IIIB according to Ann Arbor and the International Prognostic Index was 5/5. We finally confirmed that the patient with stage IIIB ULAKH had tumor lysis syndrome, bilateral chylous pleural effusion, and chyloperitoneum (Figure 3). At that time, the patient's prognosis was poor. The patient's risk of death is very high due to renal failure, heart failure, respiratory failure, depression, immunosuppression, infections and electrolyte disturbances.
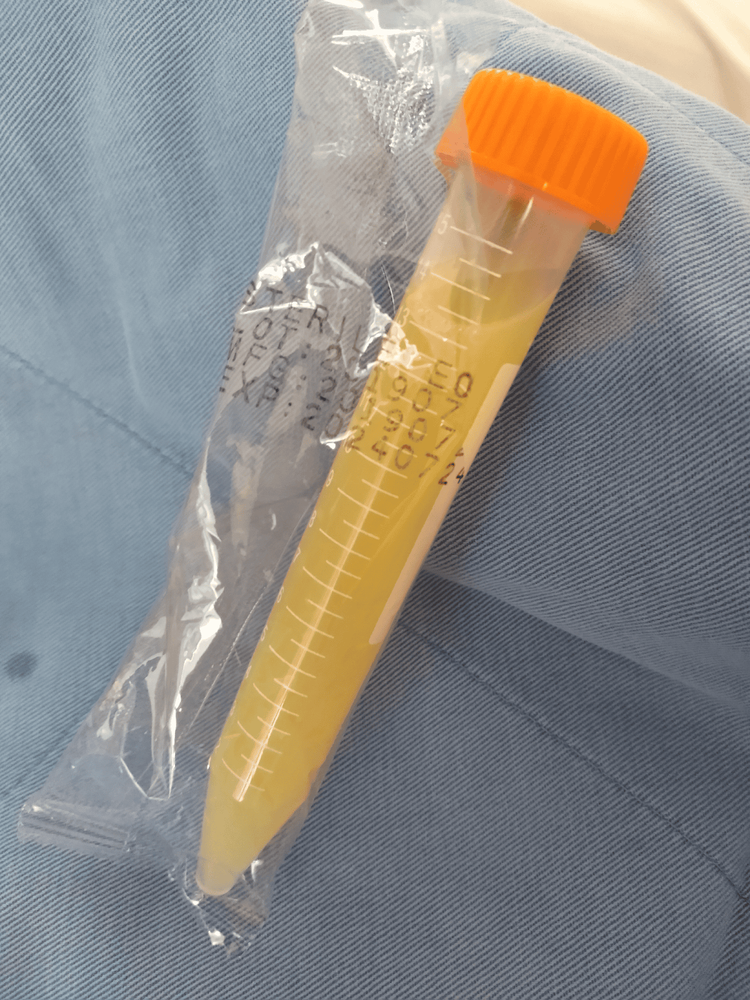
Hình 3: Hình ảnh dịch chọc hút từ ổ bụng người bệnh
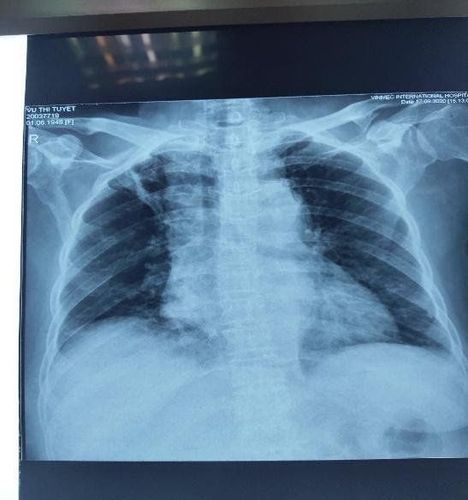
Hình 4: Chụp X quang lồng ngực sau 2 chu kỳ hóa chất
This is a late stage ULAKH disease, severe complications due to the disease, high risk of death from the time of diagnosis, but it has been accurately diagnosed, timely emergency, good treatment and initial positive results. Hopefully the treatment can help the patient live a long time.

Hình 5: Người bệnh sau 6 đợt hóa chất bước 1, phác đồ R-CVP
If you have a need for consultation and examination at Vinmec Hospitals under the national health system, please book an appointment on the website (vinmec.com) for service.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.