This is an automatically translated article.
How is a normal cell transformed into a cancer cell? Proteins involved in the regulation of cell division events no longer promote progression from one phase of the cell cycle appropriately. Instead of developing fully to perform functions, cancer cells reproduce at rates far beyond the usual tightly regulated boundaries of the cell cycle.
1. Cell cycle and cancer cell formation
Cancer can be distinguished from many other human diseases, because its root cause is not lack or decrease in cell function. For example, people with diabetes may lack insulin production or the ability to respond to insulin. With coronary artery disease, poor blood supply to the heart can cause this organ to eventually fail.
In the case of acquired immunodeficiency syndrome (AIDS), the immune system loses the cells it needs to fight infections. And with many infectious diseases, foreign microorganisms wreak havoc on the host they have invaded, causing loss of function in cells, tissues, or entire organ systems.
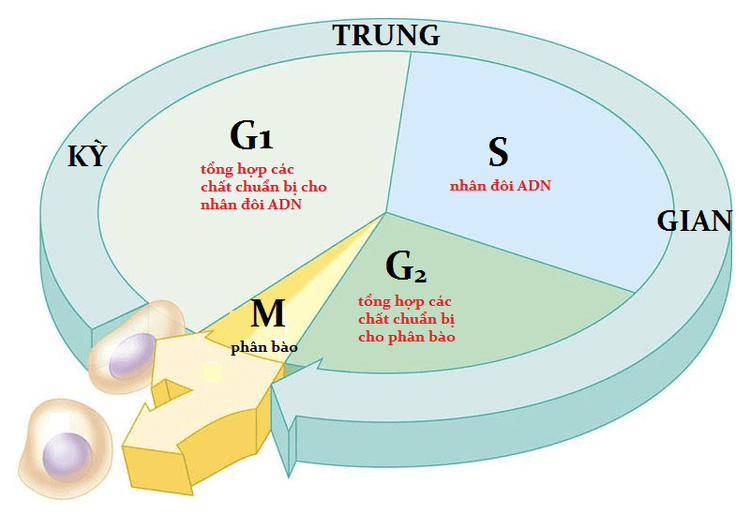
However, cancer occurs due to an alteration of a normal biological process in the body that is cell division. Cells that progress through the uncontrolled cell cycle can eventually form malignancies, where clumps of cells grow and divide uncontrollably, then develop the ability to spread and migrate move throughout the body.
Fortunately, cancer prevention often occurs through tight regulation of the cell cycle by groups of proteins that interact with each other in a very specific sequence of processes. It is these processes that determine whether the cell cycle will continue or remain stalled between phases. Given that cancer is essentially a disorder at the cellular level, advances in cell culture technology in vitro, or outside the body in general, have allowed researchers to determine which events lead to tumor formation and identify proteins involved in this process. Often called cell culture or tissue culture, researchers now routinely culture cells that are immersed in a nutrient-rich liquid also known as a medium.
Normal, non-cancerous cells will grow in a single, non-crowded layer attached to the plastic plates. These cells typically undergo a limited number of cycles of division, depending on space and nutrient availability, among other limitations. So what can happen to this orderly cell growth system? And what can be learned when things go awry? The answers to these questions form the basis for researchers' fundamental discoveries in tumor cell biology. Abnormal or cancerous cells, cultured in vitro, have been transformed from their normal phenotype due to genetic changes affecting proteins involved in cell cycle control.
These transformation tests have led to the identification of genes and proteins important for cell cycle promotion. These genes have been named oncogenes. More recently, scientists have used advances in genetic manipulation combined with transformation testing to identify genes and proteins important for limiting cell cycle progression. These genes are called tumor suppressors.
Scientists have been working to identify carcinogens and tumor suppressors and study their influence on cell cycle progression. It is even more important to understand the specific process by which multiple carcinogens and tumor suppressors must work in conjunction to promote cancer cell growth.
Các tế bào tiến triển qua chu kỳ tế bào không được kiểm soát cuối cùng có thể hình thành các khối u ác tính
2. How do cancer cells grow?
As early as 1911, Peyton Rous demonstrated through his studies of tumor growth in chickens that tumorigenesis could be transferred from one animal to another in extracts without cell. These extracts were ultimately shown to contain viruses, which have the ability to promote abnormally increased cell division in their host, serving to enhance the replication of the virus itself.
Thus, the same processes stimulated by viral replication can lead to tumorigenesis. This field of tumor virology was instrumental in the development of the "cell transformation assay" that is still used today to assess tumor growth.
How do cancer cells grow? In the test, these cells no longer required contact with the surface of the culture dish. Instead, transformed cells are capable of replicating either in agar or in suspension. This ability reflects the cancer cell's enhanced mobility, its ability to break down surrounding substances to create more space for growth and division, and reduce the contact inhibition that normally prevents these cells from spreading. cells become overcrowded.
Second, the transformed cells will grow in more than one layer, creating unusually rich cell layers. While untransformed cells grow parallel to each other in a single layer, transformed cells stack in a chaotic fashion. This feature is reminiscent of cancer cells with reduced growth inhibitor exposure.
The third characteristic of transformed cells is their requirement for fewer nutrients in the medium. This reflects the ability of tumor cells to grow and divide even in the absence of growth factors. Finally, the transformed cells overcame the restriction of the replication loops seen in normal cells and became essentially immortal.
The researchers used these results from the initial virological studies with cytological tests. In these experiments, they infected cultured cells with various viruses and then looked for "mutations" that occurred.
Because the viral genome is relatively small, researchers can easily identify the genetic components responsible for the variation. In fact, the first cancer cells they identified were of viral origin and were called viral cancers. Remarkably, the researchers also soon realized that the origin of these viral cancers came from cells that had been transferred from cell to cell by the virus.
3. Cancer cells grow out of control
What causes cancer? These are proteins involved in cell cycle regulation that work by stimulating cell growth and division. Likewise, oncogenic cells encode proteins that drive the cell cycle forward, often causing cells to proceed from one of the G (gap) phases to infectious replication. chromosomes (S phase) or chromosome segregation (mitosis).
Examples are cell surface receptors that bind to growth factors, proteins that interact with DNA to initiate replication, and signaling molecules that bind receptors to transcriptional initiators through many different paths.
In the normal state, genes that encode normal proteins that control these important processes are called proto-oncogenes. However, once they are altered to become cancerous, their abnormal protein products exhibit increased activity that contributes to tumor growth.
Thus, instead of stopping in the normal G phase, a tumor cell continues to progress through the next stages of the cell cycle, leading to uncontrolled cell division. In addition, cancer can also prevent cells from programmed cell death.
Sometimes, mutations permanently activate proteins that normally swap between active or inactive states. For example, Ras proteins act as molecular switches that are turned on and off depending on the form of nucleotide (diphosphate or triphosphate) to which it is bound.
In the "on" state, the products of these proto-oncogenes relay proliferative stimuli. However, problems arise when mutations convert the gene into an oncogene, making Ras permanently active regardless of the signal the cell receives.
The second type of genetic modification that converts genes into oncogenes is chromosomal translocations. This occurs when broken chromosome fragments reassemble indiscriminately, resulting in the formation of a fusion protein containing the N-terminus of one protein and the C-terminus of another, or resulting altered protein expression. An example of an oncogene produced by this type of chromosomal translocation is BCR/ABL, whose protein product consists of the N-terminus of Bcr (the region of the breakpoint cluster) and the C-terminus of Abl. , a tyrosine kinase that transmits proliferation signals.
The fused chromosome is called the Philadelphia chromosome, and it is widely present in patients with chronic myelogenous leukemia, a type of blood cell cancer. The formation of the fusion protein makes Abl permanently active, leading to an uncontrolled cell cycle.
However, another oncogenic vehicle does not directly alter the oncogenes at all. Instead, the proto-oncogene can exist in multiple copies in the cell, resulting in amplified expression. This is the case for c-MYC, where 8 to 30 copies are present in each HL60 cell, a leukemic cell line. Since MYC is a transcription factor, its increased expression should result in increased expression of transcriptional targets, many of which function to promote cell cycle progression towards before. Since all of these genetic alterations lead to increased function, only one affected chromosome is needed to induce the transformed cellular phenotype.

Tế bào ung thư phát triển mất kiểm soát, không giống với tế bào bình thường
4. Faulty Tumor Suppressor Doesn't Limit Cell Cycle
How are tumor suppressors different? In contrast to the proto-oncogenes and cell cycle-promoting oncogenes function, tumor suppressor genes encode proteins that normally act to limit cell growth and division. or even promote programmed cell death (apoptosis).
One of the best-studied elements of this class is a protein known as the retinoblastoma protein (pRb) and its corresponding gene RB1, the first tumor suppressor gene to be identified. Since pRb activity blocks the expression of genes required to progress into the S phase of the cell cycle, its inactivation allows uncontrolled cell division.
In fact, this principle applies to all tumor suppressors: Genetic changes in genes that lead to tumor formation prevent regulatory proteins from inhibiting cell proliferation. In other words, the growth of cancer cells cannot be stopped.
The genetic alterations that lead to pRb inactivation are often associated with frame shifts or deletions in the RB1 gene that cause premature stop codon appearance and faulty protein expression. In some cases, the expression of pRb may be normal, but the pathway in which it operates is faulty because other components of the pathway are inactive. Thus, by definition, these other ingredients would also be considered tumor suppressors.
Another example of a tumor suppressor, and the most common mutated gene in human tumours, is the p53 gene. As a transcription factor that activates the expression of proteins that inhibit proliferation and promote apoptosis in response to DNA damage, p53 plays an important role in the maintenance of the cell cycle checkpoint. G1 to S.
Genetic alterations that inactivate p53 inhibit the DNA damage response that prevents cell cycle progression. When this happens, a cell continues to divide even if the DNA is damaged. Since inactivation of tumor suppressors results in loss of function, both the original and the copies of the tumor suppressor-encoding gene often must be altered for tumorigenesis to occur.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.