This is an automatically translated article.
Article by Pharmacist Nguyen Thi Thanh Nga - Clinical Pharmacist - Faculty of Pharmacy - Vinmec Times City International Hospital
Vancomycin is the first glycopeptide antibiotic isolated from Amycolatopsis orientalis in the mid-1950s. In 1958, vancomycin was used clinically to treat penicillin-resistant Staphylococcus aureus. However, a few years later, this antibiotic was no longer preferred due to the appearance of many toxicity due to the impure preparation. After changing the manufacturing process to improve the purity of the preparation, vancomycin was again widely used since the 1980s.
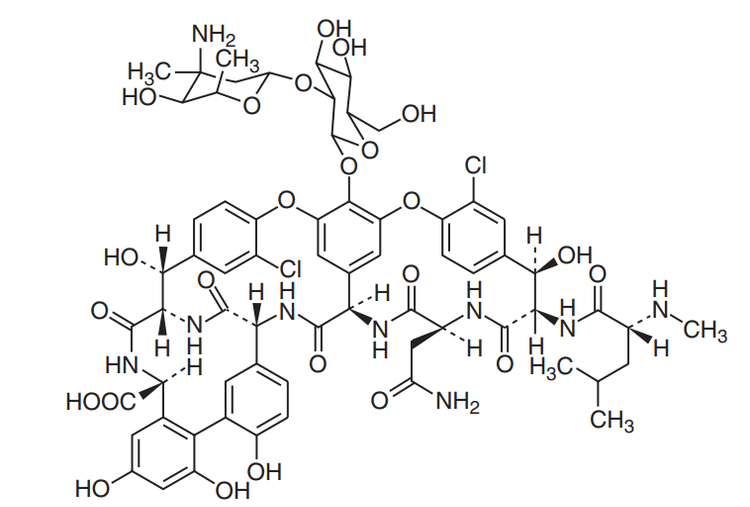
Vancomycin has a complex tricyclic glycopeptide structure consisting of 7 peptide chains attached to a disarccharide composed of 2 amino sugars (vancosamine and glucose).
1.Vancomycin pharmacokinetic model
After 1 hour intravenous infusion, blood concentrations of vancomycin generally follow a two- or three-compartment pharmacokinetic pattern. With the two-chamber model, after the end of the infusion, drug concentrations decrease rapidly due to the process of distribution from the blood to tissues (α phase, or distribution phase). After about 30 to 60 minutes of the distribution phase, drug concentrations decrease more slowly and the rate of elimination in this phase is constant, depending on renal function (β phase, or elimination phase).
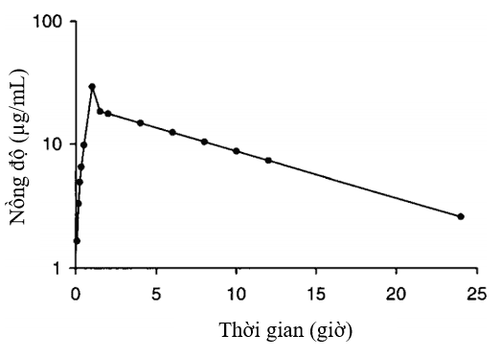
In patients with vancomycin concentrations distributed in a three-compartment pattern, there is an intermediate distribution phase between the α and β parts of the graph. However, this model is difficult to apply in clinical practice because it needs to deal with complex mathematical formulas. On the other hand, the amount of drug eliminated during infusion and waiting time for the end of the distribution phase is negligible. Therefore, a simple single-compartment pharmacokinetic model is widely used and allows accurate dose calculation when the peak concentration (Cpeak) is measured after the distribution phase. This concentration should be quantified 0.5 to 1 hour after the end of the infusion.
The pharmacokinetic model features of vancomycin are mainly based on studies in adults to find out the pharmacokinetic pattern of vancomycin in pediatric patient populations, some studies have matched the two-compartment model and Most other recent studies use only single compartment pharmacokinetic model to describe.
2. Vancomycin pharmacokinetics
2.1 Absorption Vancomycin is very poorly absorbed orally, used only in the treatment of intestinal infections caused by Clostridium difficile. Vancomycin used intramuscularly causes pain, even necrosis at the injection site, so it is not recommended. Therefore, the drug is recommended for intravenous use in the treatment of systemic infections.
2.2 Distribution Vancomycin has hydrophilic properties, so it is widely distributed into all tissues and extracellular fluids in the body, especially in young children due to the large proportion of water in the body. In non-obese adults, vancomycin's volume of distribution was 0.7 L/kg (range 0.5 to 1 L/kg). The volume of distribution (Vd) of vancomycin in children varied between populations in different studies from about 0.636 L/kg in pediatric patients not in critical care, to 0.9 L/kg in the following patients. heart surgery.
The plasma protein binding of vancomycin ranges from 30 to 60%. The ability of this antibiotic to penetrate the blood-brain barrier is very poor when the meninges are not inflamed. In children with meningitis, the drug concentration in the cerebrospinal fluid reached 14% - 28% (mean 21%) of the drug concentration in the blood after a dose of 60mg/kg/day in combination with dexamethasone, this rate was considered sufficient to kill bacteria in the cerebrospinal fluid. Vancomycin can penetrate well into pericardial fluid, pleural space, and subcutaneous and synovial tissues, but very poorly into fatty tissues [5].
2.3 Metabolism and Elimination Vancomycin is almost unchanged in the body, about 90% is eliminated by the kidneys in the active form by glomerular filtration. The vancomycin clearance rate is linearly correlated with creatinine clearance. Therefore, the recommended dose of vancomycin is adjusted based on the individual patient's renal function. Although several studies have reported a decrease in the elimination rate of vancomycin in patients with impaired liver function, the extrarenal clearance is not more than 5%. Therefore, no dose adjustment according to liver function is necessary. Clearance and half-life in children vary with age.
Neonates with immature renal function have a slow elimination rate and a prolonged half-life of vancomycin, about 5.9 to 9.8 hours in preterm infants and about 6.7 hours in neonates. full-term infants. After 3 months of age, vancomycin clearance doubles, resulting in a shortened half-life to about 4 hours. The clearance of vancomycin continues to increase and the half-life is 2-3 hours in children 4-8 years of age. After this period, vancomycin elimination and half-life are similar and reach values for adults during puberty (12-14 years).
3. Pharmacodynamic characteristics
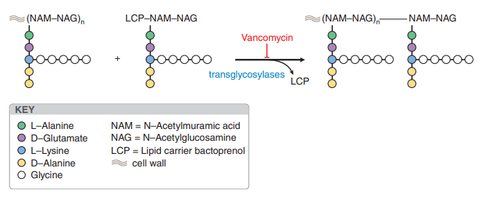
3.1 Bactericidal Mechanism Vancomycin has a bactericidal effect through inhibition of bacterial cell wall biosynthesis. Due to its affinity for binding to the D-alanyl-D-alanine terminus of the newly formed pentapeptide in the peptidoglycan chain, vancomycin inhibits the translycosylase reaction, prevents the formation of the peptidoglycan reticulum, and inhibits the synthesis of the cell wall. Due to its mechanism of action on the peptidoglycan chain, the drug is not active against Gram-negative bacteria. In addition, vancomycin affects cell membrane permeability and inhibits bacterial RNA synthesis.
Staphylococci: Staphylococcus aureus (including methicillin-resistant strains of MRSA), Staphylococcus epidermidis. Streptococcus: Streptococcus pneumoniae, S. pyogenes, S. agalactiae, S. bovis, S. mutans. Enterococci: vancomycin is bacteriostatic against most strains of Enterococcus faecalis and a certain percentage of Enterococcus faecium. Clostridium spp.: Vancomycin is active against most strains including Clostridium difficile except Clostridium ramosum. 3.3 Mechanism of resistance Enterococci Enterococci resistant to vancomycin by changing the target of action D-alanyl-D-alanin to D-alanyl-D-lactate or D-alanyl - D-serine, reducing the affinity with glycopetides, resulting in decreased inhibition of peptidoglycan synthesis. Glycopeptide resistance was most common in E. faecium (about 80%), followed by E. faecalis (about 5%) and less common in other enteric cocci.
Staphylococcus aureus The mechanism of resistance of Staphylococcus aureus to vancomycin is the thickening of the bacterial cell wall (bacteria enhance D-alanyl-D-alanin synthesis, creating false targets for staphylococcus aureus). vancomycin thereby reducing the effect of vancomycin). Thanks to that mechanism, there have now appeared strains of staphylococcus aureus that are intermediate resistant to vancomycin (VISA - Vancomycin intermediate Staphylococcus aureus), staphylococcus aureus that are resistant to vancomycin (hVISA - hetero Vancomycin intermediate Staphylococcus aureus), and staphylococci. vancomycin-resistant yellow (VRSA - vancomycin resistance Staphylococcus aureus).
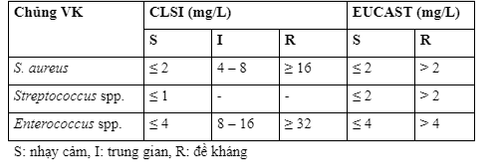
The susceptibility of bacteria to vancomycin is expressed as the minimum inhibitory concentration (MIC) parameter. The susceptibility breakpoint of vancomycin as specified by the American Institute for Clinical and Laboratory Standardization (CLSI) and the European Commission on Antibiotic Sensitivity Testing (EUCAST) is presented in the table.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References:McEvoy Gerald K, Snow Elaine K (2019), AHFS Drug Information (2019), American Society of Health-System Pharmacists, pp. Moffett Brady S, Resendiz Karla, et al. (2019), "Population pharmacokinetics of vancomycin in the pediatric cardiac surgical population", The Journal of Pediatric Pharmacology and Therapeutics , 24(2), pp. 107-116. Bauer L. A., Black D. J., et al. (1998), "Vancomycin dosing in morbidly obese patients", Eur J Clin Pharmacol , 54(8), pp. 621-5. Bauer Larry A (2008), Applied clinical pharmacokinetics, pp. 207 - 296. Bennett John E, Dolin Raphael, et al. (2015), Mandell, douglas, and bennett's principles and practice of infectious diseases , Elsevier Health Sciences, pp. 377-388. Blouin R. A., Bauer L. A., et al. (1982), "Vancomycin pharmacokinetics in normal and morbidly obese subjects", Antimicrob Agents Chemother, 21(4), pp. 575-80. Brunton L L Hilal-Dandan R, et al (2017), "Goodman and Gilman's The Pharmacological Basis of Therapeutics," pp. 1059 - 1061. Clinical and LaboratoryStandards Institute (2019), Performance standards for Antimicrobial Sucepcibility Testing, M100 , pp. 1-25. Lamarre Patrice, Lebel Denis, et al. (2000), "A Population Pharmacokinetic Model for Vancomycin in Pediatric Patients and Its Predictive Value in a Naive Population", Antimicrobial Agents and Chemotherapy , 44(2), pp. 278. Le J., Vaida F., et al. (2014), "Population-Based Pharmacokinetic Modeling of Vancomycin in Children with Renal Insufficiency", J Pharmacol Clin Toxicol, 2(1), pp. 1017-1026. Le Jennifer, Bradley John S, et al. (2013), "Improved vancomycin dosing in children using area-under-the-curve exposure", The Pediatric infectious disease journal , 32(4), pp. e155. Lietman Paul S, Schaad Urs B, et al. (1980), "Clinical pharmacology and efficacy of vancomycin in pediatric patients", The Journal of pediatrics, 96(1), pp. 119-126. Matzke G. R., Kovarik J. M., et al. (1985), "Evaluation of the vancomycin-clearance:creatinine-clearance relationship for predicting vancomycin dosage", Clin Pharm , 4(3), pp. 311-5. Moellering R. C., Jr., Krogstad D. J., et al. (1981), "Vancomycin therapy in patients with impaired renal function: a nomogram for dosage", Ann Intern Med, 94(3), pp. 343-6. Mulla H., Pooboni S. (2005), "Population pharmacokinetics of vancomycin in patients receiving extracorporeal membrane oxygenation", Br J Clin Pharmacol , 60(3), pp. 265-75. Stockmann C., Olson J., et al. (2019), "An Evaluation of Vancomycin Area Under the Curve Estimation Methods for Children Treated for Acute Pulmonary Exacerbations of Cystic Fibrosis Due to Methicillin-Resistant Staphylococcus aureus", J Clin Pharmacol , 59(2), pp. 198-205. Nang Y., Gao P., et al. (2019), "How Much Vancomycin Dose Is Enough For The MRSA Infection in Pediatric Patients With Various Degrees of Renal Function?", Iran J Pharm Res , 18(2), pp. 995-1009. WOMEN