This is an automatically translated article.
Zometa 4mg is classified as a cancer treatment drug. Zometa 4mg has the main ingredient Zoledronic Acid, which is used in the treatment of multiple myeloma, bone cancer, .... Let's learn more about Zometa 4mg through the following article.1. What is Zometa 4mg?
Zometa 4mg is classified as a cancer treatment and affects the immune system. The active ingredient in the drug is zoledronic acid.Dosage form: Zometa 4mg is prepared as a powder for infusion solution
Packing form: Box of 1 powder vial containing 4mg of zoledronic acid and 1 ampoule of solvent 5ml.
2. What does Zometa 4mg do?
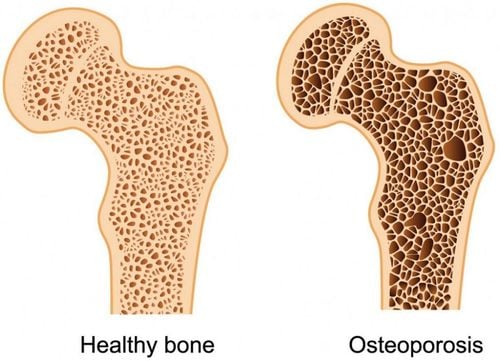
Pharmacodynamics: Zoledronic acid belongs to the group of bisphosphonates, is an acid that inhibits the release of calcium in the bones, thereby slowing bone loss as well as preventing fractures due to trauma and pathology.Indications:
Zomeat 4mg is used to treat malignant hypercalcemia (a condition in which high blood calcium levels are caused by cancer). Treatment of multiple myeloma or prevention of bone-related events (fractures, bone surgery, spinal compression,...) in patients with advanced cancer. Contraindications: Absolutely do not use Zometa 4mg in the following cases:
Patients are allergic to any ingredient of Zometa 4mg. Pregnant women, women breastfeeding. People who are taking other bisphosphonates.
3. Dosage and how to use Zometa 4mg
You need to absolutely follow your doctor's instructions about the dose and how to take the medicine. Do not arbitrarily change the dosage as well as the route of administration, do not give this medicine to others to use even if they have the same medical condition as you.Treatment of bone-related phenomena in patients with advanced cancer: 4mg Zometa in the manufacturer's solvent and further diluted for injection, infusion (can use 0.9% NaCl solution or glucose 5% Intravenous injection or infusion at least 15 minutes each time, 3-4 times. Supplement the patient with calcium and vitamin D daily.Malignant hypercalcemia: 4mg Zometa in the manufacturer's solvent and continue Continue dilution for injection, infusion (can use 0.9% NaCl solution or 5% glucose. Intravenous infusion for 15 minutes. It is necessary to provide adequate water for the patient before and during treatment.
4. Side effects
During the use of Zometa 4mg, you may encounter unwanted effects such as:Common: Leukopenia, thrombocytopenia, anemia, dizziness, headache, taste disturbances, conjunctivitis , loss of appetite, nausea, vomiting, musculoskeletal pain, body aches. Uncommon: leukopenia, sleep disturbance, anxiety, arrhythmia, hypomagnesemia, hypokalemia. The undesirable effects listed above are not exhaustive, and you may experience other unusual symptoms while taking zometa 4mg. Please report immediately to medical staff for timely assistance.
5. Drug interactions
The phenomenon of drug interactions can change the absorption, effects, and side effects of drugs. Therefore, to avoid drug interactions, you need to list and inform your doctor about all the medicines you are taking, including prescription and nonprescription drugs, dietary supplements, and herbs. In addition, alcoholic beverages such as wine, beer, ... affect the metabolism of Zometa 4mg, so you should not use these drinks while taking the drug.Incompatibilities: To avoid incompatibilities, it is necessary to dilute the drug with 0.9% NaCl solution or 5% glucose. Note that this solution must not be mixed with infusion solutions containing calcium or divalent cations (eg ringer lactate), and set up a separate infusion line, not with other drugs.
6. Some notes when using Zometa 4mg
Pregnant and breastfeeding women: Zometa 4mg is contraindicated. Assess the patient's general condition before administering the drug to ensure adequate hydration. Avoid excessive fluid intake with heart failure patients. Closely monitor metabolic indices related to hypercalcaemia such as serum calcium, phosphate, and magnesium concentrations after the first dose. If hypocalcaemia, hypophosphataemia, hypomagnesemia, additional treatment is required. Patients with untreated hypercalcaemia are at risk for renal failure, so renal function should be monitored. Do not take Zometa 4mg with aclasta concurrently. There are insufficient data on the safety of the drug in children. Patients with Renal Impairment: The benefits and risks should be weighed when deciding to treat with Zometa 4mg in patients with renal impairment. Serum creatinine should be assessed prior to each dosing. When the drug is used in patients with bone metastases with mild to moderate renal impairment, the dose should be reduced. In patients with evidence of renal damage during treatment, the drug should be reintroduced when creatinine levels return to within 10% of baseline. Patients with hepatic impairment: There is insufficient clinical evidence in patients with hepatic impairment, so the use of this drug is not recommended.7. Preservation of drugs
Store Zometa 4mg in a sealed package, in a dry place, away from mold and direct sunlight, at a temperature below 30 degrees Celsius. Keep the medicine out of reach of children and pets.Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.