This is an automatically translated article.
Belonging to the cardiovascular group, Vesup Tablet is indicated for the prophylaxis of thromboembolic disorders such as myocardial infarction, peripheral artery disease or for the control and prevention of recurrence in patients with recent coronary artery disease. stroke.
1. What are the effects of Vesup Tablet?
1.1. What is Vesup Tablet? Vesup Tablet with registration number VN-19208-15, manufactured by Samjin Pharma Co., Ltd - Korea.
Vesup Tablet contains the following ingredients:
Main active ingredient: Clopidogrel bisulfate equivalent to Clopidogrel 75mg. Excipients: Lactose monohydrate, Hydroxypropylcellulose less substitute for L-HPC, Sodium stearyl fumarate, Silicone dioxide glue and Opadry Pink (03B84893). The drug is prepared in the form of 75mg film-coated tablets, blisters of 10 tablets, boxes of 3 blisters. Vesup Tablet is recommended for adult use.
1.2. What diseases does Vesup Tablet treat? Clopidogrel is a prodrug. One of its metabolites is a platelet aggregation inhibitor.
Vesup Tablet is prescribed by doctors in the following cases:
Hospital thrombosis: Adult patient with myocardial infarction (from a few days to less than 35 days), ischemic (from 7 days to less than 6 months) or confirmed peripheral arterial disease. Patients with acute myocardial infarction: No ST-segment elevation (unstable angina or non-Q-wave myocardial infarction), use in combination with acetylsalicylic acid (ASA) or ST-segment elevation , combined with ASA in the treatment of thrombolysis .
Prevention of arterial and vascular thrombosis in atrial fibrillation. Vesup Tablet is contraindicated in the following cases:
Patients are allergic to the main active ingredient Clopidogrel or any of the excipients of the drug. Patients with severe liver failure. The patient is having problems with pathological bleeding such as peptic ulcer, bleeding in the brain.
2. Usage of Vesup Tablet
2.1. How to take Vesup Tablet Vesup Tablet is taken orally. Take Vesup Tablet with a sufficient amount of filtered water (about 200ml). Do not mix Vesup Tablet with any other mixture. Strictly follow the recommendations for use or instructions of the doctor 2.2. Dosage of Vesup Tablet Recommended dosage: 75mg/time/day.
Patients with acute myocardial infarction without ST-segment elevation (unstable angina or non-Q-wave myocardial infarction):
Start treatment with 300mg Vesup Tablet on the first day, after Then continue with a dose of 75mg / time / day (in combination with acetylsalicylic acid 75 – 325 mg / day) in the following days. Taking high doses of ASA may lead to a higher risk of bleeding, so doses of ASA should not exceed 100 mg. The optimal duration of treatment has not been formally established. Clinical trial data show that the treatment lasts for 12 months and the most effective effect is after 3 months. Patients with acute myocardial infarction with ST-segment elevation:
Initial dose of 300mg Vesup Tablet, then 75mg/time/day, in combination with ASA. In elderly patients (over 75 years of age), do not use the starting dose. Combination therapy should be initiated as soon as possible, preferably as soon as symptoms appear, and for a duration of at least 4 weeks. In patients with atrial fibrillation, a dose of 75 mg once daily is recommended. Initiate and prolong therapy in combination with ASA (75-100mg/day). Handling when missed dose:
If you forget to take a dose but not more than 12 hours later than the time to take it: The patient takes 1 tablet immediately and then takes the next pill at the scheduled time to take the medicine. If 1 dose is missed, more than 12 hours: Skip the missed dose and take the next dose. Do not take a double dose to make up for missed doses. Treatment of overdose:
Overdose when taking Clopidogrel can cause prolonged bleeding and complications after bleeding. Appropriate treatment should be given when signs of bleeding are present. There is no specific antidote. Platelet transfusion may help limit the effects of clopidogrel.
3. Notes when using Vesup Tablet
Due to the risk of hematologic activity and adverse events, a complete blood cell count should be performed prior to treatment or a variety of tests should be performed when clinical bleeding is detected during treatment. with Vesup Tablet. Vesup Tablet should be used with caution in patients at risk for hypertension, surgery, or in patients being treated with ASA, heparin, Ilb/Illa glycoprotein inhibitors and non-steroidal anti-inflammatory drugs (NSAIDs). including Cox-2 inhibitors. Patients should inform their doctor that they are taking clopidogrel before starting treatment with a new drug. Clopidogrel prolongs bleeding time and should be used with caution in patients with a potential for bleeding (especially gastric, intestinal and intraocular). Increased risk of bleeding due to trauma, surgery, liver failure or other medical conditions. Vesup Tablet should be discontinued 7 days before surgery. Do not use Vesup Tablet for the first 7 days after local blood loss. Vesup Tablet should not be used concurrently with oral thrombolytic drugs that may increase the degree of bleeding. Vesup Tablet should be used with caution in pregnant and lactating women because there have not been many studies on its use in this group of subjects.
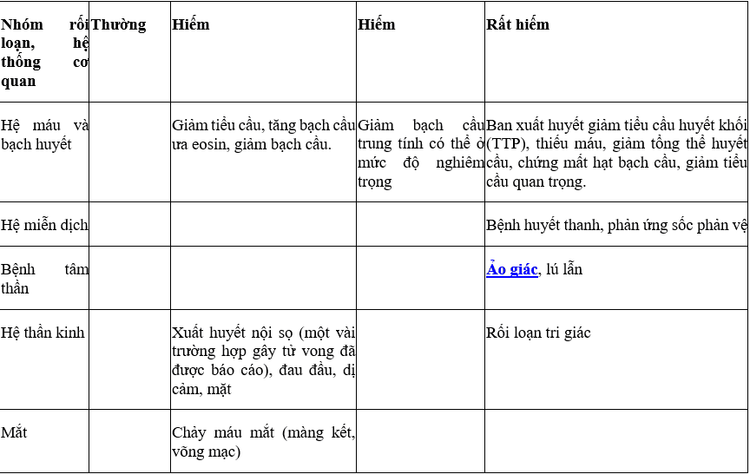
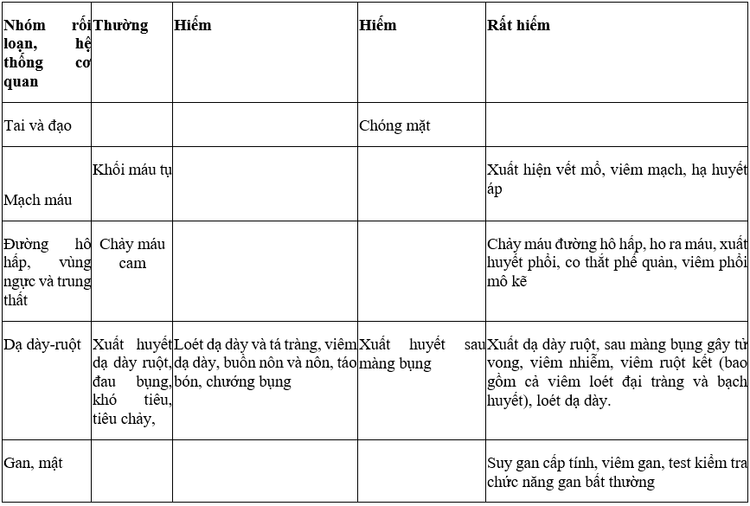
4. Vesup Tablet side effects
Bleeding during the first month of treatment is a frequently reported accessory application in clinical studies.
Some other side effects like:
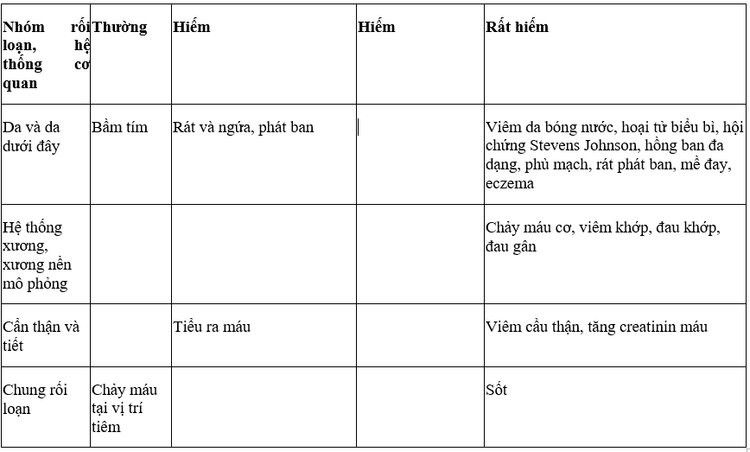
5. Vesup Tablet drug interactions
Vesup Tablet should not be used in combination with warfarin as it may increase the degree of bleeding. Caution should be exercised when clopidogrel and ASA are used concurrently. The combination of 500mg ASA twice daily did not increase bleeding time due to Vesup Tablet. Vesup Tablet should be used with caution in combination with NSAIDs including Cox-2 inhibitors, as there have been no studies on whether this combination increases the risk of bleeding from the gastrointestinal tract. Caution should be exercised when Vesup Tablet is used concomitantly with strong or moderate CYP2C19 inhibitors, including: Omeprazole and esomeprazole, fluvoxamine, fluoxetine, moclobemide, voriconazole, fluconazole, ticlopidine, ciprofloxacin, cimetidine, carbamazepine, oxcarbazepine and chloramphenicol. Because inhibitors of CYP2C19 reduce the activity of clopidogrel. The pharmacokinetics of digoxin or theophylline were unchanged when co-administered with clopidogrel. Gastric antacids do not alter the absorption time of clopidogrel. To avoid interactions, before being prescribed Vesup Tablet, patients should inform their doctors about all the drugs they are using, including functional foods. The doctor will base on that to prescribe the appropriate Vesup Tablet.
6. How to store Vesup Tablet
The shelf life of Vesup Tablet is 36 months from the date of manufacture. Store Vesup Tablet in a cool, dry place, away from direct light, at a temperature not exceeding 30°C, in its original packaging. Keep Vesup Tablet out of reach of children. The article has provided information on dosage, contraindications and notes during use and Vesup Tablet's therapeutic uses. To ensure that Vesup Tablet works optimally and to prevent side effects, patients should consult their doctor/pharmacist before use.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.