This is an automatically translated article.
Mirzaten 30mg is a type of quadricyclic antidepressant, effective in treating major depressive episodes, helping to improve mood, balance psychological well-being, bring a feeling of comfort and well-being. Read more articles below to understand more about what mirzaten is and the uses of mirzaten.
1. What is mirzaten 30mg?
Mirzaten has the main ingredient Mirtazapine, which is made in the form of 30mg film-coated tablets, belonging to the group of antidepressants. Each tablet contains the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, titanium dioxide, and red iron oxide.
Absorption of the drug mirzaten Mirzaten tablets 30mg are rapidly and completely absorbed after oral administration and have a half-life of approximately 20 to 40 hours. Peak plasma concentrations occur approximately 2 hours after oral administration.
Metabolism of the drug Mirzaten Mirzaten 30mg is extensively metabolized after oral administration. The main biotransformation pathways of the drug are hydroxylation and demethylation, followed by glucuronide conjugation. Studies in human liver indicate that cytochromes 1A2 and 2D6 are involved in the formation of the 8-hydroxy metabolite of Mirzaten, while the cause of formation of the N-desmethyl and N-oxide metabolite is cytochrome 3A. Mirzaten 30 mg has an absolute bioavailability of about 50%. It is eliminated mainly in the urine (75%) with 15% in the faeces. Presence in plasma at very low levels are several pharmacologically active unconjugated metabolites. The (-) enantiomer has a half-life approximately two times longer than the (+) enantiomer and thus achieves plasma concentrations three times higher than that of the (+) enantiomer. .
Elimination of the drug Mirzaten Mirzaten 30mg is extensively metabolised and eliminated in the urine and faeces within a few days, with a mean half-life of 20-40 hours depending on age and sex, with women of all ages having the half-life is significantly longer than in men (mean half-life is 37 hours for women compared to 26 hours for men); Longer half-lives, up to 65 hours, have been reported occasionally and shorter half-lives have been observed in young men. The half-life of the drug is justified for once-daily dosing. Steady state is reached after 3-4 days, after which there is no further accumulation.
Mirtazapine is approximately 85% bound to plasma proteins over a concentration range of 0.01 to 10 mcg/mL.
The clearance of mirtazapine may be reduced due to renal or hepatic impairment.

Thuốc mirzaten 30mg là thuốc nhóm chống trầm cảm
2. The uses of the drug mirzaten 30mg
2.1. Mechanism of action of mirzaten 30mg Mechanism of action Mirzaten Tablets 30mg of action is evidenced in preclinical studies that it enhances the activity of the central noradrenergic and serotonergic systems. These studies have shown that mirtazapine acts as an antagonist at central presynaptic α2-adrenergic inhibitory receptors and heteroreceptors, an action thought to lead to increased noradrenergic activity and central serotonergic.
Mirtazapine is a potent antagonist of 5-HT 2 and 5-HT 3 receptors. Mirtazapine has no appreciable affinity for 5-HT 1A and 5-HT 1B receptors.
Mirtazapine is a potent antagonist of histamine (H 1) receptors, a property that may explain its prominent sedative effects.
Mirtazapine is a moderate peripheral α1-adrenergic antagonist, a property that may explain the infrequently reported orthostatic hypotension associated with its use.
Mirtazapine is a moderate antagonist at muscarinic receptors, a property that may explain the relatively low rate of anticholinergic side effects associated with its use.
2.2. Indications of the drug mirzaten 30mg Mirzaten tablets 30mg are indicated for the treatment of major depressive disorder. The efficacy of Mirtazapine Tablets in maintaining response in patients with major depressive disorder for up to 40 weeks after initial 8 to 12 weeks of open-label therapy was demonstrated in a controlled trial with placebo.
2.3. Contraindications of the drug mirzaten 30mg Do not use the drug in any patient who is allergic to any of its ingredients.
Use of monoamine oxidase inhibitors (MAOIs) for the treatment of psychiatric disorders with Mirtazapine Tablets, USP is contraindicated, or within 14 days of stopping treatment with Mirtazapine Tablets because of the increased risk of serotonin syndrome .
2.4. Undesirable effects of mirzaten 30mg Children Mirzaten 30mg should not be used in the treatment of children and adolescents under 18 years of age. Behaviors related to suicide (suicide attempts and thoughts of suicide) and hostility (primarily aggression, oppositional behavior, and emotional incontinence) appear more frequently in clinical trials in children and adolescents treated with antidepressants compared with those treated with placebo. However, if treatment is still decided based on clinical need, the patient should be carefully monitored for the appearance of suicidal symptoms.
Suicide/suicidal thoughts or medical worsening Depression is associated with an increased risk of suicidal thoughts, self-harm, and suicide (suicide-related events). This risk persists until the disease tends to be in significant remission. Patients should be closely monitored during the first weeks of treatment because psychological improvement has not been evident. During the early stages of recovery, the risk of suicide may be increased. Patients with a history of suicide or suicidal ideation prior to initiation of treatment are at higher risk and should be carefully monitored. kidneys during treatment. Close monitoring of patients and especially high-risk patients is required during antidepressant treatment, especially at early initiation and after dose modification. Patients (and caregivers) should be alerted to the need to monitor for any clinical deterioration, suicidal ideation or behavior, and unusual changes in behavior and to seek medical attention. Get medical advice immediately if these symptoms are present. For the possibility of suicide, especially at the start of treatment, only a limited number of Mirzaten 30mg film-coated tablets should be given to patients. good patient management, to reduce the risk of overdose.
Serious skin reactions Serious skin adverse reactions (SCAR) including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), drug reactions with eosinophilia and systemic symptoms (DRESS), bullous dermatitis and erythema multiforme, which can be life-threatening or fatal. If signs and symptoms suggestive of these reactions occur, the drug should be discontinued. right away. If a patient has developed one of these reactions while using Mirzaten 30mg, this drug should not be used again.
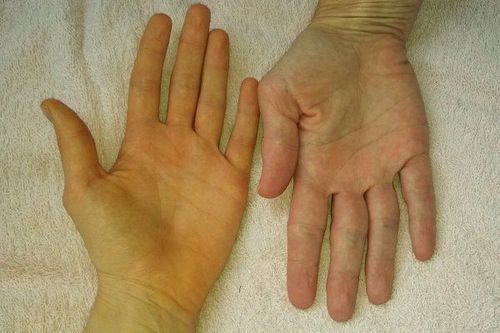
Bệnh nhân bị vàng da do dùng thuốc mirzaten 30mg nên ngừng sử dụng ngay
Jaundice The drug should be discontinued if jaundice occurs.
Some other manifestations: Careful dosing as well as frequent and close monitoring is necessary in patients:
Epilepsy and organic brain syndrome: Treatment should be discontinued in any patient seizures or where there is an increased frequency of seizures. Hepatic impairment: Following an oral dose of 15 mg mirtazapine, clearance of mirtazapine was reduced by approximately 35% in patients with mild to moderate hepatic impairment, compared with subjects with normal hepatic function. Mean plasma concentrations of mirtazapine increased by approximately 55%. Renal Impairment: Following a single oral dose of mirtazapine 15 mg, in patients with moderate (creatinine clearance <40 ml/min) and severe (creatinine clearance ≤10 ml/min) renal impairment, clearance of mirtazapine was approximately 30% and 50% reduction compared with normal subjects. Mean plasma concentrations of mirtazapine were increased by approximately 55% and 115%, respectively. There were no significant differences in patients with mild renal impairment (creatinine clearance <80 ml/min) compared with controls. Heart conditions such as conduction disturbances, recent angina attacks, and myocardial infarctions, require common-sense precautions and careful concomitant medication administration. Low blood pressure. Diabetes: In patients with diabetes, antidepressants may alter glycemic control. Dosage adjustment of insulin and/or oral hypoglycemic agents may be required and close monitoring is recommended. Hyponatremia Hyponatremia, possibly due to inappropriate secretion of antidiuretic hormone (SIADH), has been reported very rarely with the use of mirzaten 30 mg. Caution should be exercised in patients at risk, such as elderly patients or patients receiving concomitant therapy with drugs known to cause hyponatremia.
Serotonin syndrome Interaction with serotonergic substances: serotonin syndrome may occur when selective serotonin reuptake inhibitors (SSRIs) are used concurrently with other serotonergic active substances. Symptoms of serotonin syndrome may include hyperthermia, rigidity, myoclonic tremor, autonomic instability with rapidly fluctuating vital signs, mental status changes including confusion, irritability extreme agitation and agitation leading to delirium and coma. Caution and closer clinical monitoring are required when these active ingredients are combined with mirtazapine. Treatment with mirtazapine should be discontinued if such events occur and symptomatic supportive treatment initiated.
Lactose This medicinal product contains lactose. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicine.

Bệnh nhân cao tuổi cần lưu ý khi dùng thuốc mirzaten có thể gây hạ natri máu
2.5. Drug interactions of mirzaten 30mg Using mirzaten 30mg together with the following drugs may reduce the effect of that drug, so it is necessary to inform your doctor about the medicine you are taking while being treated with mirzaten 30mg
Inhibitors Monoamine Oxidase Inhibitors Serotonergic Drugs Drugs Metabolized by and/or Cytochrome P450 Enzymes Inhibitors When Phenytoin, carbamazepine, or another inducer of hepatic metabolism (e.g. rifampicin) are used together with mirzaten 30 mg, the dose of mirtazapine may need to be increased. . Cimetidine Ketoconazole Amitriptyline Warfarin Lithium Risperidone Alcohol Diazepam QTc Prolonging Drug Caution is advised when mirtazapine is co-administered with strong CYP3A4 inhibitors, HIV protease inhibitors, azole antifungals, erythromycin or nefazodone.
3. How to use mirzaten 30mg
Adults The effective daily dose is usually 15mg to 45mg; The starting dose is 15mg or 30mg. Mirzaten 30mg generally begins to work after 1-2 weeks of treatment. Treatment with appropriate dosage will give positive results within 2-4 weeks. If an adequate response is not obtained, the dose may be increased up to the maximum dose. If there is no response within another 2-4 weeks, then treatment should be discontinued.
Patients with depression should be treated for a sufficient period of at least 6 months to ensure that they are symptom-free.
Treatment Mirzaten 30mg has a half-life of 20-40 hours and therefore mirzaten 30mg tablets are suitable for once-daily administration. It is best taken as a single dose at night before bedtime. Mirzaten 30mg tablets can also be given in two divided doses (once in the morning and once at night, the higher dose should be taken at night).
The tablets should be taken orally, with liquid and swallowed without chewing.
Sử dụng thuốc mirzaten 30mg theo đúng chỉ định của bác sĩ
4. Notes when taking mirzaten 30mg
As with other antidepressants, users of mirzaten 30mg should also be aware of:
Worsening of psychotic symptoms may occur when antidepressants are administered to patients with schizophrenia or other disorders. other psychotic; Paranoid thoughts may be intensified When the depressive episode of bipolar disorder is being treated, it may turn into a manic episode. Patients with a history of mania/mania should be closely monitored. Mirzaten 30 mg should be discontinued in any patient entering a manic episode. Although mirzaten 30mg is not addictive, post-marketing experience shows that abrupt cessation of treatment after long-term use can sometimes lead to withdrawal symptoms. The majority of withdrawal reactions are mild and self-limiting. Among the reported withdrawal symptoms, dizziness, agitation, anxiety, headache, and nausea were the most frequently reported. Although they have been reported as withdrawal symptoms, it should be recognized that these symptoms may be related to pre-existing medical conditions. Caution should be exercised in patients with movement disorders such as prostatic hypertrophy and in patients with acute narrow-angle glaucoma and ocular hypertension (although there is little chance of problems with mirtazapine because of its active activity). its very weak anticholinergic properties). Akathisia/psychotic restlessness: Antidepressant use has been associated with the development of akathisia, which is characterized by an unpleasant or subjectively distressing feeling of restlessness and frequent need to move accompanied by an inability to move. sit or stand still. This is most likely to happen during the first few weeks of treatment. In patients who develop these symptoms, increasing the dose may be detrimental. Cases of QT prolongation, torsades de pointes, ventricular tachycardia and sudden death, have been reported during postmarketing use of mirzaten 30 mg. The majority of reports occurred in association with overdose or in patients with other risk factors for QT prolongation, including concomitant use of QTc-prolonging agents. Mirtazapine should be used with caution in patients with known cardiovascular disease or a family history of QTc prolongation, and when co-administered with other medicinal products thought to prolong the QTc interval.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.