This is an automatically translated article.
Derinide 200 Inhaler is an inhaler used in the treatment of bronchial asthma, containing the main active ingredient is Budesonide - a glucocorticosteroid with high anti-inflammatory activity. So what are the dosages, side effects and precautions when using Derinide 200 Inhaler?1. Uses of Derinide 200 Inhaler
Each bottle of Derinide 200 Inhaler contains 40mg Budesonide, equivalent to 200mcg Budesonide in 1 dose. Budesonide is a glucocorticosteroid with high anti-inflammatory activity. The mechanism of action may be due to increased number and activity of anti-inflammatory cells, inhibition of airway smooth muscle contraction, and decreased airway hyperresponsiveness. Inhaled corticosteroids are fast acting locally and have limited systemic side effects.Derinide 200 Inhale r is indicated in the treatment of bronchial asthma.
Derinide 200 Inhaler is contraindicated in the following cases: Patients with hypersensitivity to Budesonide or any other ingredients of the drug; relieve symptoms of acute bronchospasm ; Initial treatment in persistent asthma or acute asthma attacks requires emergency measures.
2. Dosage and usage of Derinide 200 Inhaler
2.1. Amount
The dosage of Derinide 200 Inhaler will depend on the severity of the disease and the individual characteristics.Children: The recommended dose is 50-100mcg x 2 times/day. Dosage may be increased to 800 mcg/day depending on symptoms and patient response. Adults: Treatment should be initiated at a dose of 400 - 1600 mcg/day, divided into 2 - 4 times/day. For maintenance therapy, the dose should be adjusted based on individual patient response and the lowest possible dose for symptom control should be used. Maintenance dose is usually in the range of 200-400 mcg x 2 times / day, used in the morning and evening. After a single dose, Derinide 200 Inhaler has an effect of several hours, the full therapeutic effect is achieved only after a few weeks of treatment.
2.2. How to use
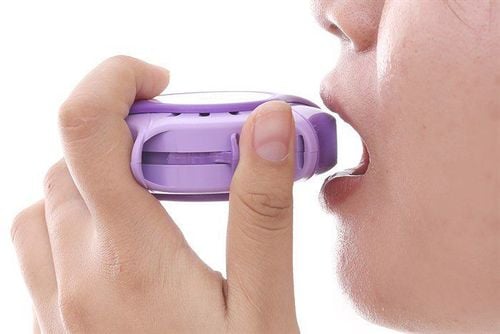
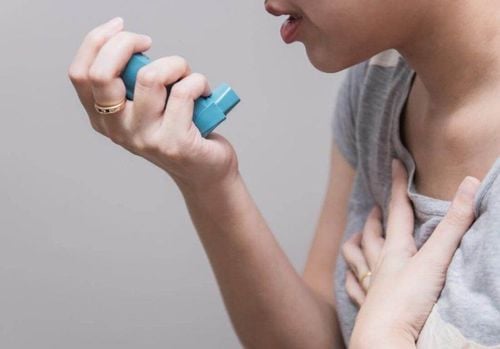
Instructions on how to use the Derinide 200 Inhaler potion:B1: Remove the protective cap and check the potion. Shake well to mix the medicine in the bottle. B2: Hold the potion with your thumb and index finger. Breathe gently through your mouth and place the tip of the potion in your mouth between your teeth, taking care not to bite into the potion. B3: Close your lips around the mouthpiece, tilt your head back slightly. Inhaling slowly while pressing the bottle to release a dose of medicine, continue to inhale slowly and deeply. Step 4: Remove the potion from your mouth. Hold your breath for about 10 seconds and then exhale slowly. B5: If the patient needs an additional dose, shake the bottle again and repeat steps 1 - 4. B6: Rinse mouth thoroughly with water after each use to avoid oropharyngeal fungal infection. Note: It is important to press down on the dose and inhale deeply. This allows as much of the drug to get into the lungs as possible. Clean the bottle by washing the plastic part regularly (2 times a week), remove the bottle and wash the plastic part in warm not too hot water with mild detergent if necessary. Allow the resin to dry completely and insert the Derinide 200 Inhaler bottle.
3. Undesirable effects of the drug Derinide 200 Inhaler
Patients using Derinide 200 Inhaler may experience the following symptoms:Mild irritation in the throat, voice disturbances. Insomnia, excitement. Candidiasis in the pharynx - pharynx, rhinitis, otitis media. Skin rash, pruritus, hypersensitivity reactions. Nausea, vomiting, abdominal pain, digestive disorders. Osteoporosis, broken bones. Weight gain. May cause bronchospasm in hyperresponsive patients. Bronchospasm can be treated with a bronchodilator beta 2 agonist. Patients can be prevented by taking a beta 2 agonist 5 to 10 minutes before taking Derinide 200 Inhaler . Rarely, patients may develop symptoms of systemic glucocorticoids, including adrenal insufficiency. Bruising has also occurred in some patients.
4. What are the precautions when using Derinide 200 Inhaler?
To minimize the risk of candida infection in the mouth/throat, patients should rinse their mouth with water after each use. Concomitant administration of Derinide 200 Inhaler with ketoconazole and other strong CYP3A4 inhibitors should be avoided. If co-administration is required, the above drugs should be used with Derinide 200 Inhaler as far as possible. Caution should be exercised when treating patients switching from systemic corticosteroids to Derinide 200 Inhaler and in cases of suspected pituitary-adrenal dysfunction. In these patients, it is prudent to reduce the dose of systemic steroids and check the hypothalamic-pituitary-adrenal axis function. Patients also require additional systemic steroids during periods of metabolic stress such as surgery, trauma, etc. During the transition from systemic steroid therapy to Derinide 200 Inhaler, the patient may re-emerge in symptoms. have had before such as muscle and joint pain. In these cases, a temporary increase in the dose of oral steroids may be necessary. Patients who have replaced systemic steroid therapy with Derinide 200 Inhaler also occasionally develop signs of an allergic reaction, eg rhinitis, eczema. In rare cases, if symptoms such as fatigue, headache, nausea, vomiting or similar symptoms occur, inadequate steroid action may be suspected. Vasculitis: Rare cases of vasculitis or systemic eosinophilic conditions may occur. This condition is usually associated with reduction and/or discontinuation of oral corticosteroid therapy after initiation of inhaled corticosteroids. Effects on bone mineral density: Use with caution in patients with risk factors for decreased bone mineral density such as prolonged immobility, family history of osteoporosis, menopausal status, Tobacco use, advanced age, poor nutrition, or chronic use of medications can reduce bone density. Pregnancy: In pregnant animals, inhaled budesonide has been associated with fetal development abnormalities, but relevant evidence has not been found in humans. Therefore, Derinide 200 Inhaler should be avoided during pregnancy unless absolutely necessary. Lactation: Budesonide is present in breast milk. According to the manufacturer, the decision to breastfeed during treatment should weigh the risks and benefits of the drug. Inhaled budesonide is considered compatible with breastfeeding5. Drug interactions Derinide 200 Inhaler
Ketoconazole at a dose of 200 mg once daily increased plasma concentrations of budesonide by a mean of 6-fold when co-administered. There is no information on this interaction for inhaled budesonide, however significantly increased plasma concentrations may also occur. Therefore, the combination should be avoided. If a combination must be used, the distance between Derinide 200 Inhaler and Ketoconazole should be as far as possible. In addition, other strong CYP3A4 inhibitors (erythromycin, clarithromycin, calcium channel blockers, etc.) can also significantly increase the plasma concentration of budesonide.The article has provided an overview of the drug Derinide 200 Inhaler. Some patients are treated with Derinide 200 Inhaler but still have no control of their symptoms due to incorrect technique. Therefore, patients should consult a doctor or pharmacist to ensure the best effect of the drug.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.