This is an automatically translated article.
Posted by Master, Doctor Mai Vien Phuong - Head of Department of Gastrointestinal Endoscopy - Department of Medical Examination & Internal Medicine - Vinmec Central Park International General Hospital
How probiotics can affect different conditions is still poorly understood, but several mechanisms have been suggested. Accordingly, Probiotics have been studied to have a positive effect on gastrointestinal diseases.
1. Some mechanisms on the role of probiotics in digestive diseases
How probiotics can affect different conditions is still poorly understood, but several mechanisms have been suggested. One is that the gut microbiota influences visceral pain and sensitivity, and the expression of mu-opioid and cannabinoid receptors induced by Lactobacillus in the intestinal epithelium may reduce pain in a manner similar to opioids.
Another proposed mechanism is the regulation of the immune system. Several studies have found that probiotics or their products block inflammatory cytokines and stimulate protective cytokines, most in inflammatory bowel disease (IBD) models. Finally, probiotics may promote intestinal epithelial integrity, protect intestinal epithelial tight junctions and barrier function, and may induce biofilm secretion of factors that can inhibit entry of pathogens.
As an understanding of the gut microbiome and the complex interactions involved in inflammation, intestinal permeability, and metabolic dysfunction, the potential of probiotics is appealing to clinicians and patient. However, while enthusiasm for probiotics has skyrocketed, especially along with other highly advertised nutritional supplements, little data supports their use. Perhaps more importantly, the gastrointestinal diseases for which they have benefits and the species that confer these benefits are still unclear, leading to confusion between clinicians and the general public.
This evidence-based review is an update of a 2018 review intended to guide selection of probiotic regimens, where the data appear robust and highlight areas that need further research before it can be established. make more concluding recommendations.
2. Looking for evidence
Methods used to find recommendations regarding searching through PubMed, OVID and the Cochrane Review Library for the terms “probiotics”, “indications”, “dose”, “inflammation” bags”, “infectious diarrhea”, “antibiotic-associated diarrhea”, “Constipation”, “irritable bowel syndrome”, “hepatic encephalopathy”, “ulcerative colitis”, “Crohn’s disease” and "Clostridioides." Results were further narrowed to randomized controlled trials (RCTs), meta-analyses, and review articles where information on specific strains and dosages can be found. Proposals are selected based on the relative certainty of the data involved.
3. Some treatment regimens of Probiotics in clinical practice
Selected Probiotic Mode
| Viêm túi | Dự phòng ban đầu: VSL # 3 3 g / ngày trong 12 tháng |
| Tiêu chảy nhiễm trùng | Lactobacillus casei GG 6 × 10 9 CFU hai lần mỗi ngày trong 5 ngày |
| Tiêu chảy do liên kết với Clostridium difficile | L. acidophilus 25 × 10 9 CFU / ngày trong 2 ngày, sau đó 50 × 10 9 CFU / ngày trong thời gian dùng kháng sinh HOẶC L. casei 19 × 10 9 CFU / ngày, L. bulgaris 1,9 × 10 9 CFU / ngày, và Streptococcus thermophilus 19 × 10 9 CFU / ngày, tất cả đều bắt đầu trong vòng 48 giờ sau khi dùng kháng sinh và tiếp tục trong 7 ngày sau khi dùng kháng sinh |
| Helicobacter pylori nhiễm trùng | Dự phòng điều trị tiêu chảy liên quan: Lactobacillus GG hai lần mỗi ngày? Trong suốt thời gian điều trị và trong 7 ngày sau đó |
| Táo bón | L. casei Shirota 6,5 × 10 9 CFU / ngày trong 4 tuần HOẶC E. coli Nissle 1917 25 × 10 9 CFU / ngày trong 8 tuần |
| Hội chứng ruột kích thích | Bifidobacterium Infantis 35624 1 × 10 9 CFU / ngày trong 4 tuần HOẶC B. bifidum MMBb 75 1 × 10 9 CFU / ngày trong 4 tuần |
| Bệnh não gan | Không có chế độ khuyến nghị |
| Bệnh Crohn | Không có chế độ khuyến nghị |
| Viêm loét đại tràng | Escherichia coli Nissle 1917 200 mg / ngày để duy trì sự thuyên giảm |
3.1. Helicobacter pylori infection
Another antibiotic-related indication for probiotics is for patients undergoing treatment for the eradication of Helicobacter pylori. In an updated, evidence-based international consensus, experts concluded that probiotics are useful as adjunctive therapy to prevent or reduce the duration or intensity of AEs to eradication therapy. H. pylori and improve adherence. 20 In a randomized, placebo-controlled study, patients on a standard regimen supplemented with H. pylori probiotics reported lower rates of cardiovascular events, including diarrhea and overall treatment tolerability is improved. 21 Probiotics given during and for 7 days after H. pylori therapy; patients received one of several effective regimens, including Lactobacillus GG twice daily, S. boulardii twice daily, or a combination of Lactobacillus and Bifidobacterium administered twice daily.
A meta-analysis of 10 clinical trials of adjuvant probiotics in patients with H. pylori infection demonstrated increased cure rates with probiotic supplementation (synthetic odds ratio [OR) ], 2.07; 95% CI, 1.40-3.06) and reduced the incidence of total antibiotic-related AEs (synthetic OR, 0.31; 95% CI, 0.12- 0.79).
3.2. Constipation
Use of probiotics in patients with severe chronic constipation and without irritable bowel syndrome (IBS) has also been studied and the results are inconclusive. A randomized, double-blind, placebo-controlled study found that a probiotic beverage contained L. casei Shirota at a dose of 6.5 × 10. Although small studies have suggested improvement bowel movements, including frequency, stool consistency and intestinal transit time with Bifidobacterium lactis DN-173 010, B. lactis BB12,
3.3. Irritable bowel syndrome
Although data supporting probiotics for IBS are generally limited largely by methodological shortcomings, there is some support for their use for variant diarrhea (IBS-D). Controlled trials have shown that B. Infantis 35624 at a dose of 1 × 10 8 CFU daily for 4 weeks can improve abdominal pain, bloating, bowel dysfunction, and incomplete bowel movements. , tension and air conduction. 25 One B. bifidum MIMBb75 capsule at a dose of 1 × 10 9 CFU for 4 weeks effectively reduced IBS globally and concurrently improved IBS symptoms, with a subjectively reported improvement in quality of life. Studies examining several species of Lactobacillus, including LPO1, and L. plantarum 299V, as well as B. bifidum MIMBb75, B. breve BR, and VSL
3, have also shown improvement in symptoms reported by patients. reported, including gas and bloating, but did not show an overall effect on stool quality or frequency.
ACG monograph on the Management of Irritable Bowel Syndrome, published in 2018, suggested probiotics can be used to improve global symptoms as well as abdominal distention and flatulence in patients with IBS patients.
A more targeted microbiome approach is evolving. A systematic review of the gut microbiota in IBS described a decrease in the genus Bifidobacterium. To address this specific biological disorder seen in IBS, a randomized, double-blind, placebo-controlled study explored human milk oligosaccharides (HMOs), specifically a 4:1 mixture. of 2'-O-fucosyllactose and lacto-N-neotetraose and found that it increased the abundance of Bifidobacterium species in IBS patients. In summary, moderate or severe IBS-related symptoms were significantly improved after 12 weeks in adults who consumed supplements containing HMOs, with an overall 55% reduction in IBS severity scores. This type of precision microbiological modification therapy will grow in the coming years.
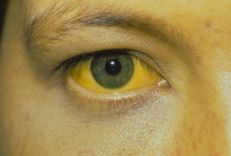
3.4. Hepatic encephalopathy
In various forms of chronic liver disease, the liver - secondary to injury and redirection of blood flow - loses its ability to remove ammonia from the body. Increased levels of ammonia lead to a reversible brain disease known as hepatic encephalopathy. Treatment is aimed at increasing excretion or reducing ammonia production. Lactulose - a pro-biotic, acting through ion traps along with a strong laxative effect. Rifaximin (Xifaxan, Salix) works by reducing the population of urease-producing bacteria. In theory, probiotics work by making the gut environment more favorable for bacterial species that do not produce urease or by regulating the pH of the intestinal lumen, thereby reducing ammonia production and preventing or reversing disease. liver brain.
One RCT has shown that at a daily dose of 3 capsules containing 112.5 billion lyophilized viable bacteria per capsule, each capsule contains 4 strains of Lactobacillus (L. casei, L. plantarum, L. acidophilus). and L. delbrueckii subspecies bulgaricus ), 3 strains of Bifidobacterium ( B. longum , B. breve , and B. infantis ), and 1 strain of Streptococcus salivarius (subspecies thermophilus ), which were more effective than no secondary prophylaxis. clinical hepatic encephalopathy and was as effective as standard lactulose therapy. Overall, however, no mortality benefit has been identified to support the use of probiotics alone in the treatment of hepatic encephalopathy.
Recently, the focus has shifted to analyzing the efficacy of fecal microbiota transplantation (FMT) in patients with hepatic encephalopathy. A study published in the journal Hepatology found that FMT reduced hospital admissions and improved cognitive performance and physiological dysfunction in cirrhotic subjects with recurrent hepatic encephalopathy. In addition, FMT capsules were shown to be safe and associated with improvement of duodenal mucosal diversity, dysbacteriosis, reduction of lipopolysaccharide-binding protein, and improved performance of EncephalApp. Further research is needed to determine the best technique targeting the microbiome to ameliorate hepatic encephalopathy.
While the suggested health benefits of probiotics continue to trend on social media and remain an active area of clinical research, there is clearly a shift towards other modalities of microbiome-based therapy. With confounding variables such as heterogeneity in study design, many researchers have begun to focus on other ways to induce changes in the microbiome, including diet, prebiotics, and prebiotics. and FMT. In the near future, microbiome therapy will become more targeted and personalized, and precise microbiome control will become a reality.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
ReferencesBrown AC, Valiere A. Probiotics and Medical Nutrition Therapy. Nutr Clin Care . 2004; 7 (2): 56-68. Rousseaux C, Thuru X, Gelot A, et al. Lactobacillus acidophilus modulates intestinal pain and produces opioid and cannabinoid receptors. Nat Med. 2007; 13 (1): 35-37. Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by analysis of the gut microbiota of patients with Crohn's disease. Proc Natl Acad Sci USA . 2008; 105 (43): 16731-16736. McCarthy J, O'Mahony L, O'Callaghan L, et al. Double-blind, placebo-controlled trial of two rat probiotic strains interleukin 10 and its mechanical association with cytokine balance. Intestine . 2003; 52 (7): 975-980. https://www.gastroendonews.com/