This is an automatically translated article.
Post by Master, Doctor Ma Van Tham - Head of Pediatrics - Neonatology Department - Pediatrics - Neonatology Department - Vinmec Phu Quoc International General Hospital
Stool test to diagnose amoebic dysentery is being used commonly. In some cases where it is not clear, doctors will order more colonoscopy, blood tests, ELISA methods, PCR to support the diagnosis.
1. Stool microscopy in diagnostic test for Amoebic dysentery
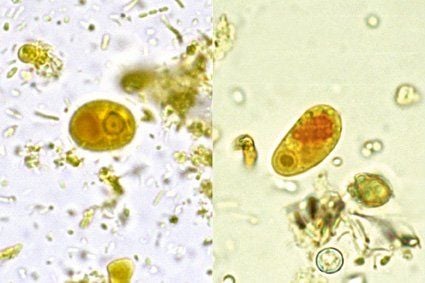
Demonstration of active cysts or cells in the stool suggests intestinal infection, but microscopy cannot distinguish between strains of E. histolytica and E. dispar or E. moshkovskii. In addition, microscopy requires specialized expertise and is subject to operator error.
The excretion of organisms can vary, at least 3 specimens on separate days should be submitted to detect 85-95% of infections. Specimens can be concentrated and stained with iodine to detect cysts. To find the active forms, it should be bound with saline and stained with iron hematoxylin and/or trichrome; immobilized with polyvinyl alcohol.
Stool samples are often positive for blood in the setting of invasive intestinal infections. The presence of erythropoiesis is not predictive of E. histolytica infection ; There are not always white blood cells in the stool because white cells can be destroyed by the organism.
2. Antigen test
Antigen detection is sensitive, specific, rapid, easy to perform and can differentiate between E. histolytica and E. dispar . Stool and serum antigen detection tests use epithelium-binding monoclonal antibodies present on pathogenic E. histolytica strains (but not on nonpathogenic E. dispar strains). Antigen detection kits using enzyme-linked immunosorbent assay (ELISA), radioactivity or immunofluorescence assay have been developed. Antigen detection has many advantages, including the ease and speed of testing, the ability to differentiate between strains, greater sensitivity than microscopy, and the ability to diagnose early and latent infections. endemic areas.
Several new tests with different reported sensitivity and specificity have been developed. The TechLab E. histolytica stool antigen test is an ELISA test specific for E. histolytica . Gal/GalNAc lectin detection assay as determined by E. histolytica in stool samples; it has a sensitivity of 87% and a specificity of >90% compared with culture. A study comparing the TechLab E. histolytica specific antigen detection test with PCR assay showed comparable sensitivity when performed directly on fresh stool samples.
3. Molecular method for the diagnosis of amoebic dysentery
Probe detection of parasitic DNA or RNA in stool can also be used to diagnose infections and to distinguish between 3 different strains.
Some PCR tests that can detect E. histolytica in stool samples are available. These include conventional PCR, nested PCR, real-time PCR, multiplex PCR, and loop-mediated isothermal amplification assays. Most PCR tests have 100% sensitivity and specificity, PCR is about 100 times more sensitive than stool antigen tests. In addition, PCR can distinguish between pathogenic and non-pathogenic infections, and multiple PCR tests allow simultaneous detection of multiple pathogens.
4. Serological diagnosis of dysentery Amoebiasis
Infection with E. histolytica leads to the development of antibodies; E. disar infection no. Antibodies can be detected within 5 to 7 days of acute infection and can persist for years. Approximately 10 - 35% of uninfected individuals in endemic areas have antibodies against bacteria due to previous E. histolytica infection. Thus, seronegative helps rule out disease, but seropositivity cannot distinguish between acute infection and previous infection.
Indirect hemolysis (IHA) is the most sensitive serological test, it is positive in approximately 90% of patients with symptomatic enteric infections. Diffusion and agar gel reflexes are less sensitive than IHA but usually only remain positive for 6 to 12 months, which may make them more useful in endemic areas. A commercially available ELISA with 93% sensitivity compared to IHA has also been developed.

5. Colonoscopy in diagnostic test for Amoebic dysentery
Diagnostic test for amoebic dysentery can be done by colonoscopy. However, colonoscopy is not suitable as a routine diagnostic tool because the presence of bacterial ulcers increases the likelihood of perforation during the introduction of air to dilate the colon.
The cecum and colon are the most common sites of involvement. In one study, endoscopic findings of colitis were observed in the cecum, rectum, ascending colon, transverse colon, sigmoid colon, and descending colon (93, 45, 28, 25, 20). and 15%, respectively).
Biopsies are preferably taken from the edge of the ulcer, which may be positive for cystic or active microscopy and antigen testing for E. histolytica may be positive. Colonic involvement in amoebic dysentery ranges from thickening and nonspecific mucositis to classic spherical amoebic ulcers.
Diagnostic testing for amoebic dysentery is usually done by stool microscopy, stool antigen testing, or stool PCR. Fecaloscopy is laborious with limited sensitivity but is the simplest method available that is easy to perform with high specificity.
Antigen and PCR tests are more sensitive and can differentiate between E. histolytica and E. dispar infections. PCR has the highest sensitivity and is the ultimate diagnostic tool, but high cost is a barrier to its use as a routine test in disease diagnosis.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.