This is an automatically translated article.
The article was written by a doctor of Biochemistry - Laboratory Department - Vinmec Ha Long International HospitalLiver cancer is the most common cancer, ranking 5th in men and 7th in women. Early detection is highly effective in treatment. Ultrasound and Tumor markers recommended for screening for early detection of liver cancer are now available, including AFP, PIVKAII (DCP) and AFP-L3. The following article summarizes the meaning and usage of these tests.
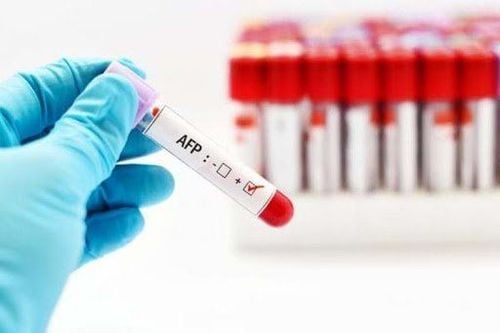
1. AFP
AFP stands for A lpha1- f eto p rotein, which is a single-chain glycoprotein with a molecular weight of 70000 daltons. AFP is synthesized mainly in the embryonic liver and yolk sac, then into the amniotic fluid, crosses the placenta into the maternal blood. In pregnant women AFP is used in a trio of tests including AFP, beta hCG and uE3 to screen for the risk of abnormalities in chromosomes 13, 18 and 21 in the baby (Triple test, performed at gestational week) 16-18). When the baby is born, AFP levels drop rapidly to normal levels (0-7 ng/mL). In the nonpregnant person AFP is used to screen for the diagnosis of germ cell cancer (eg, testicular cancer) and liver cancer.There have been many studies on AFP and made recommendations on the use of AFP test in liver cancer screening such as: In 2009, Asian cancer scientists (AOS) and European Cancer Society (European Cancer Society) ESMO) has agreed to recommend serum AFP levels every 3-6 months for all cases of cirrhosis - especially hepatitis B cirrhosis for cancer screening; In 2020, the US National Cancer Network (NACB) recommended screening for liver cancer in people at high risk, especially cirrhosis due to hepatitis B and C, with AFP quantification and abdominal ultrasonography. 1 time / 6 months. If AFP levels are abnormal and ultrasound shows a nucleus < 1cm, it is necessary to monitor and test again after 3 months. If AFP is >20 ng/mL and gradually increases, it is important to monitor (even if ultrasound results are normal) by increasing the frequency of testing. Abnormal AFP levels should be combined with other tests such as PIVKA II and AFP-L3 to increase the sensitivity and specificity for diagnosis. If AFP level > 200 ng/mL and nucleus > 2 cm, then treat without further biopsy.
2. AFP-L3
AFP comes in three forms: AFP-L1 is the predominant form seen in people with benign liver disease (eg, chronic hepatitis B or cirrhosis; AFP-L2 is produced by yolk sac tumors; AFP- L3 is produced by malignant hepatocytes, which is the predominant form seen in patients with primary liver cancer (HCC-H epato c ellular C arcinoma).Units used for AFP-L3 are % ( % of AFP-L3/AFP The recommended cut-off value is <10%.According to Marrero et al. (2009), AFP-L3 is 92% specific at any stage of cancer. liver cancer, but the sensitivity is only 37%, when the amount of AFP is < 20 ng/ml, AFP-L3 cannot be detected.
3. PIVKA II (or DCP)
DCP stands for D es-gamma- c arboxy-p rothrombin, PIVKA II stands for P rothrombin i nduced by vitamin K a bsence II, is an abnormal form caused by a lack of prothrombin vitamin K.Mechanism of formation of DCP or PIVKAII: During carcinogenesis of hepatocytes, the prothrombin-dependent carboxylase system is damaged so it cannot do the carboxylation task leading to DCP production, and DCP loses prothrombin function
AFP, PIVKAII (DCP) và AFP-L3 là 3 loại xét nghiệm phát hiện sớm ung thư gan
In primary hepatocellular carcinoma (hepatom-HCC), the mean concentration of DCP was found to be 900 ng/mL.
Currently, AFP is the most widely used and widely used marker, because it is available to most hospitals and is reasonably priced to be used every 6 months in people at risk. (Chronic hepatitis B, C virus, cirrhosis due to hepatitis B, C). If liver cancer is suspected or advanced, the test should be performed at a frequency of ≤ 1 time / 3 months and should combine the AFP, AFP-L3 and PIVKA II tests.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
Reference source: Hoang Van Son. Screening, early detection and diagnosis of liver cancer. Journal of Vietnamese Medicine, vol. 496, November-thematic issue, 2020 and other internet sources.