This is an automatically translated article.
The article was made by a doctor of Laboratory - Vinmec Central Park International General Hospital
There are many comparisons of serological techniques listed in the article, it is probably best to acknowledge what can be done in general, but to add any technique is very much dependent on the specific agent. circumstances and conditions that cause the disease. In general, there is a trend towards standardization, automated execution of large numbers of specimens, and point-of-care testing.
There are advances in both simple and complex serological diagnostic tests based on developments in science. It is clear that experience creates methodological models for use in serological diagnosis for the following agents. There has been a significant influence from the development of serological diagnosis of viral diseases because the field is inherently difficult to detect viruses. Trends and problems illustrated by a detailed review of the serological diagnosis of Mycoplasma pneumoniae served as a template for the development of other fields in bacteriological diagnosis (34).
1. Bacterial agglutination
This method is the easiest among the simple methods. The primary bacterial suspension was inactivated and the densities were normalized to be viewed with the naked eye after reacting with the patient serum dilution series. Aggregation can be performed in tubes or in wells (35). The color of the bacterial suspension can enhance reaction observations. Aggregation is caused by interactions between antibodies and antigens on the bacterial surface. The antigen-antibody complex is quite large when the bacterial protozoa are attracted.
This method is applicable to bacteria that grow extracellularly and are of sufficient size to show turbidity in the suspension. The method is insensitive when compared with most - if not all - current methods. However, good standardization of these types of tests can mitigate this (36).
2. Diffusion (immunoassay) stable . test
This method is essentially an agglutination test but is convenient because of its stability on a hard medium (such as agarose) to see the reaction. Antigen is diffused from a starting point (such as a well), and is necessarily different from typical bacterial agglutination because the antigen must be smaller in order to move through the rigid rack. Antigens can be derived from many bacterial products, from cell walls to intracellular products. The antigen-antibody interaction is represented by an agar precipitate between the antigen initiation point and the antibody initiation point. Antigen and antibody diffusion is passive and thus migration occurs in all directions from the original well. When moving the antibody will combine with the antigen, but many antigens and antibodies have a partial non-interaction and they move by centrifugal force. This reduces the sensitivity of the test and it is of little use in bacteriological diagnosis. The nature of the reaction also does not quantify the antigen-antibody response, although on the run the magnitude of the ribbon is indicative of the amount of this interaction. This test requires a period of 18-24 hours and the reaction tape should be visible after staining.
The interaction between antigens and antibodies can be focused using a gentle electric current that will bring those components into direct opposition (convection immunoelectrophoresis: counter immunoelectrophoresis; CIE) (37) . This makes for a fast reaction rate, ensuring that antigen-antibody contact is maximized. However, CIE is also not as sensitive as current methods (38) and is currently only used for antigen detection rather than antibody detection.
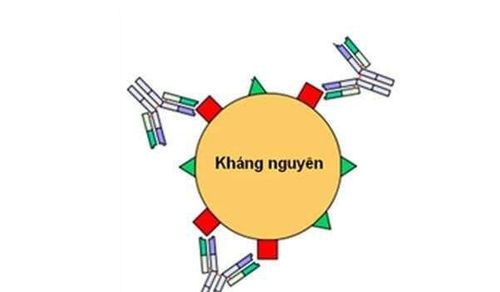
CIE hiện nay chỉ sử dụng để phát hiện kháng nguyên
3. Carrier particle agglutination
The above methods rely on visual observation of the antigen-antibody interaction which is not conspicuous, thus tending to induce some form of increased expression of the reaction. Antigen attachment to large racks makes it easier to observe the reaction.
There are many rigid supports for this purpose, including latex beads, gelatin molecules, red blood cells (3-5% suspension)....(39-41). Normally, antigen binds to the scaffold passively, in the case of red blood cells the conjugation can be active if it alters the erythrocyte surface (e.g. treatment with tannic acid). The latex particles used here are <10 μm in size. All these supports and its binding to the antibody are relatively stable, the reagent can be kept for many months. The test can be performed on slides or equivalent rigid stands or on tubes or wells. These tests are rapid in nature, requiring only dilution of the serum prior to testing.
The reaction can detect both IgG and IgM, although indirect hemagglutination (IHA) is more often IgM. The titer of the IHA test is necessarily dependent on response. Maximal IgM response, and better response in younger patients, such as children and young adults, than older adults with less IgM responders.This test is a likely method for rapid diagnosis. of the IHA is close to that of other methods, such as the EIA.
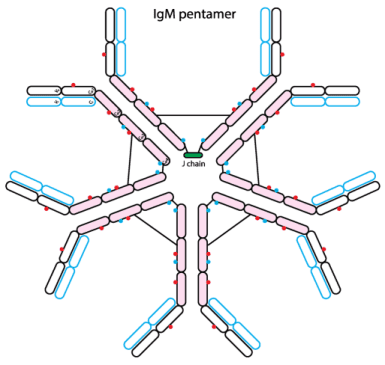
Thử nghiệm có thể phát hiện được cả IgM
However, a nonspecific reaction can occur, by agglutination with the scaffolding particles rather than with the bacterial antigen, which occurs in about 5-10% of the tests (42). For example, antibodies can pick up many different antigens on the surface of red blood cells. Therefore, it is also necessary to control this problem. Furthermore, the agglutination reaction itself is difficult to read in some dilutions, especially at the breakpoint of the antigen-antibody interaction (suspected). When only one dilution is used, suspicious results can occur in about 1-5% of cases depending on the antigen carrier molecule used.
A variation of this method is the red blood cell-IgM capture assay. In this test, antibodies to the μ-chain of IgM bind to red blood cells. Red blood cells are the visible carrier. The diluted sera series is mixed with the IgM-conjugated carrier, and the standard antigen is added last (43).
4. Growth inhibition or neutralization
Antibodies that inhibit bacterial enzymatic activity or bacterial growth, both of which can be exploited for serological diagnostic purposes. For the former, bacterial enzymes or exotoxins can be neutralized with a serum dilution prior to a detection test. Anti-streptolysin O and serum antiDNAase assays in Streptococcus pyogenes infections mostly use this method (6, 44). This requires partially or completely purified microbial ingredients. This method is clearly based on all infectious bacterial strains capable of producing products that induce an antibody response to those products as virulence factors.
Neutralization of the infectious agent is synonymous with growth inhibition. Diluted sera are reacted with infectious agents, and these agents are then cultured. Many methods are commonly used in virological diagnosis, but are also used to measure protective antibodies for a number of purposes (45). Antibodies can inhibit bacteria in the presence or absence of complement. Measure inhibition by monitoring directly or indirectly by monitoring the lack of bacterial products, such as metabolic inhibition and pH indicator color change (46). This method detects IgG better than IgM, although IgG, IgM, and IgA all inhibit bacterial components and thus peak antibody concentrations often occur later in disease progression than with other methods. mainly detects IgM. These tests usually take more than a day. There may be a low titer due to antibody cross-reactivity produced in response to other bacteria. If the patient is taking antibacterial drugs, there may be falsely high titers due to the inhibitory or bactericidal drug.
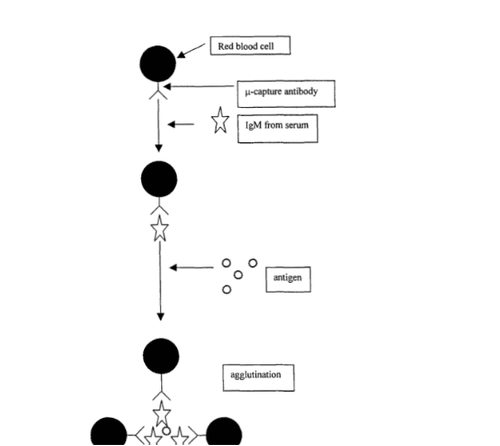
Hình 1: Thử nghiệm hồng cầu tóm bắt IgM (the red blood cell-IgM capture assay)
5. Complement fixation
There was a desire to increase the sensitivity of serological diagnostic tests, and this was achieved in part by the proliferation of antigen-antibody response detection systems. Furthermore, trends in serological diagnosis provide the basis for this concern. In complement fixation (CF), complement fixation of the antigen-antibody reaction is exploited (47). When antibody levels are increased as a result of infection, there is a greater possibility of complement fixation in the presence of immobilized antigens. Complement fixation was determined by a biologically effective complement detection system. In the classical CF test, sensitized red blood cells used as an indicator of complement immobilization (suggesting the presence of less antibodies) are combined with antibody lysates that react with red blood cells. and cause red blood cell lysis. If complement combines with the antigen-antibody complex, it will not lyse red blood cells. The observation of intact and lysed red blood cells in the test well will indicate the final antibody dilution that reacts (Figure 2).
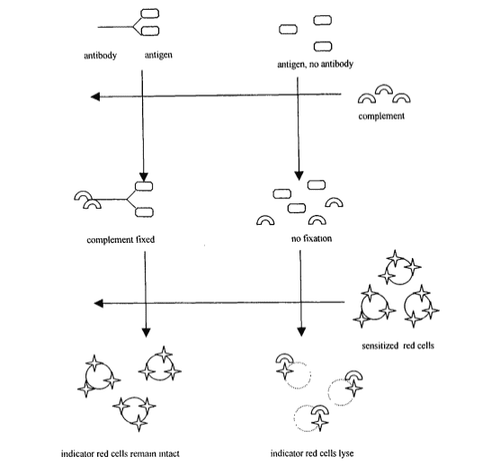
Hình 2: Thử nghiệm cố định bổ thể
The CF test needs to be standardized and rigorously tested, but good quality reagents are also available commercially (48). The key changes in test performance are particularly in complement source, quality and source of hemolysin, appropriate red blood cells and antigens. The preparation of the ingredients as well as the execution of the test took more than a day. However, the CF test can be configured to assess the response to more than one antigen in the form of a panel test and by using the same detection system. The CF test is usually done in a reference laboratory.
The traditional CF test is often used as a benchmark against which other test methods are compared. Antibodies, especially IgM, are usually detected during the acute phase, although both IgM and IgG are complement fixative (48). A titre above the threshold or a quadruple increase between acute and remission sera is used for diagnosis. The sensitivity of the CF test is roughly the same as that of the EIA test but EIA is preferred due to its simplicity of implementation. For some infections, sensitivity may be suboptimal, such as the CF test for chlamydiae.
Some sera may have anti-complement activity that complicates the test, and this should be detected by appropriate controls. Serum anti- complement may occasionally be seen in Q fever (49).
Invite you to follow the document on Serological Diagnosis of Infections by Doctor Tran Thi Ngoc Anh including:
Serological Diagnosis of Infections Antigens and antigenic variation Antigen relationship Voidness specificity of the humoral immune response Summary of serodiagnostic methods - Part 1 Summary of serodiagnostic methods - Part 2 General aspects of utilization in hematology Bar The Complicated Situation in Serological Diagnosis Source: Nevio Cimolai
Children's and Women's Health Center of British Columbia, Vancouver, British Columbia, Canada