This is an automatically translated article.
Posted by Doctor Vu Duy Dung - Department of General Internal Medicine - Vinmec Times City International Hospital
Many serious neurological complications can occur after Subarachnoid hemorrhage (SAH) such as re-bleeding, hydrocephalus, seizures, cerebral ischemia...
1. Re-bleeding
Rebleeding is the most common immediate life-threatening neurological complication following SAH. The best way to reduce the risk of rebleeding is to treat an unsealed ruptured aneurysm promptly. Prevention of rebleeding through intensive blood pressure control should begin immediately during prehospital transport and in the emergency department.
2. Hydrocephalus
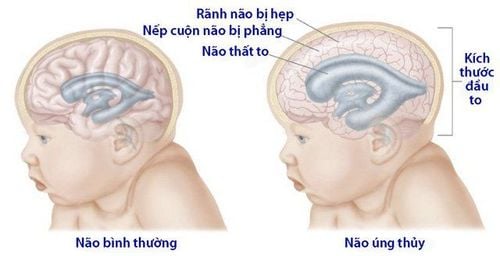
Tràn dịch não hay còn gọi là não úng tủy vẫn có thể xảy ra ở bệnh nhân SAH
Symptomatic acute hydrocephalus occurs in 20% of patients with SAH, usually within minutes to days after the onset of SAH. Clinical signs of hydrocephalus are decreased consciousness, decreased ability to gaze upward, increased blood pressure, and delirium. Diagnosis is confirmed by clinical symptoms and head CT scan.
Hydrocephalus is self-correcting in 30% of patients but can also worsen rapidly. Insertion of an external ventricular drain (EVD) can save the patient's life. Some centers place a lumbar drain instead of an EVD in cases of hydrocephalus, while some centers place both types of drainage. Constraints to placement of an EVD include the risks of infection, bleeding (intracerebral or intraventricular), and transmural pressure variations that pose a risk of rebleeding an unobstructed aneurysm. The risk of bleeding and infection with EVD insertion is close to 8% for each type.
Rapid withdrawal of the EVD is recommended after aneurysm removal or within 48 hours of drainage if the patient is neurologically stable. In patients whose drainage cannot be removed (approximately 40%), placement of a long-term ventricular-peritoneal drain may be necessary. A small retrospective study in Germany suggested that dexamethasone 12 mg/day for at least 5 days may reduce the risk of hydrocephalus after SAH. Due to the lack of randomized controlled studies, the routine use of corticosteroids other than indicated for the management of meningeal chemotaxis headache after SAH is not recommended.
3. Seizures and seizure prevention

Cơn động kinh ở bệnh nhân SAH
Determining the true incidence of seizures in patients with SAH is difficult because many patients (up to 26%) present with epileptic seizures, but these seizures are not easy to define when they occur at a specific time. point of onset of symptoms. If seizures occur prior to aneurysm occlusion, they are often a sign of early rebleeding. Persistent seizures are seen in 2% of patients with SAH and are associated with a greater severity of SAH. Rates of nonconvulsive seizures (7% to 18%) and nonconvulsive status epilepticus (3% to 13%) are more common in SAH patients who are comatose and associated with cerebral ischemia. late and worse outcomes. It is not completely understood whether nonconvulsive seizures are the cause of late ischemia and poor outcome or a phenomenon of severe SAH with poor outcome due to the severity of SAH. Because nonconvulsive seizures are treatable, ongoing EEG monitoring should be considered in patients with severe SAH. A 2015 review summarized nonconvulsive seizures and nonconvulsive status epilepticus in SAH. However, in some hospitals, the limitation lies in the ability to perform and read prolonged continuous EEG. Furthermore, recent findings suggest that surface EEG can detect nonconvulsive seizures in only 8% of SAH patients, whereas they are present in 38% of patients when EEG is measured via deep electrodes. in the cortex is placed through a hole in the skull. Such deep recording electrodes, however, are more invasive, have limited use in very few centers, and are not currently considered standard of care. Nonconvulsive status epilepticus should be considered in comatose SAH patients.
Currently, in the absence of randomized controlled clinical trials of antiepileptic drug therapy in patients with SAH, antiepileptic drug therapy should be limited to preoperative treatment of aneurysms. , examines the known adverse effects of antiepileptic drugs, especially phenytoin, on neurocognitive recovery after SAH.
Guidelines and experts recommend stopping antiepileptic drugs in patients who can be reliably monitored clinically after the aneurysm has been sealed and not to prolong seizure prophylaxis beyond 3 to 7 days if the disease is present. patients without seizures at onset of SAH. At the author's hospital, only comatose patients and patients with severe SAH were continued on antiepileptic drugs after aneurysm surgery because of the increased risk of nonconvulsive seizures in these patients. this.
Levetiracetam is a commonly used antiepileptic drug because of its high bioavailability, favorable adverse event profile, and absence of drug interactions. It should be noted, however, that no studies have shown an advantage of levetiracetam over other antiepileptic agents. In addition, levetiracetam has not been approved by the US Food and Drug Administration (FDA) for epilepsy monotherapy; therefore, no specific antiepileptic drug can be recommended for seizure prevention in patients with SAH.
4. Late cerebral ischemia
Thiếu máu não cục bộ muộn là một trong những biến chứng thần kinh
Late ischemic stroke is one of the most worrisome neurological complications after SAH, as late ischemic cerebral infarction is the leading cause of disability in SAH survivors. Late ischemic monitoring is the primary reason for recommending long-term intensive care unit (ICU) stay in patients with SAH. Late cerebral ischemia is defined as any neurological deterioration that persists for more than 1 hour that cannot be explained by other neurological or systemic disorders, such as fever, seizures, hydrocephalus. encephalopathy, infection, hypoxia, sedation, and other metabolic causes. Late cerebral ischemia is diagnosed when other causes of neurologic deterioration have been excluded or are not sufficient to cause neurodegeneration and is therefore a diagnosis of exclusion.
Historically, late cerebral ischemia was thought to be caused by cerebral vasospasm. However, current evidence indicates that the pathogenesis of late cerebral ischemia includes an interaction of early brain injury, microthrombosis, diffuse cortical depolarization, associated ischemia, and constriction. cerebral vasoconstriction. Furthermore, some experts believe that cerebral vasospasm is only a side effect and that the biochemical and biochemical changes leading to late cerebral ischemia occur at the time of SAH onset. This underpinning change in the approach to late ischemia is supported by a negative endothelin 1 antagonist trial in SAH patients who were clipped or coiled.
Endothelin 1 has been implicated as the most potent vasoconstrictor mediator in SAH. However, the administration of clazosentan, a potent inhibitor of the endothelin 1 receptor, resulted in a reduction in cerebral vasoconstriction but no improvement in late cerebral ischemia and no improvement in outcome 3 months after SAH.
Late cerebral ischemia occurs on average 3 to 14 days after SAH. The risk of late ischemic stroke is increased with thickened SAH and intraventricular bleeding, as illustrated by the modified Fisher score. Additional risk factors include clinical severity, loss of consciousness at onset, smoking, cocaine use, SIRS, hyperglycemia, hydrocephalus, and nonconvulsive seizures. Predicting which patients will develop late ischemic stroke has proven to be difficult but very important. Not only will such prediction have an impact on ICU follow-up, early diagnosis, and treatment, but also on resource availability and early ICU exit for patients with mild and low-risk SAH. . The best predictors of patients requiring less close follow-up include advanced age (over 65 years), low WFNSS scores 1 to 3, and modified Fisher scores less than 3.
5. Prophylaxis of late cerebral ischemia

Thuốc Nimodipine giúp bảo vệ thần kinh
Calcium channel blockers (nimodipine) and maintenance of normal intravascular fluid status have the strongest evidence for intervention in the prevention of late ischemia. Nimodipine (60 mg every 4 hours for 21 days) is neuroprotective and has Class I evidence for a reduced risk of adverse functional outcomes. However, it did not reduce the frequency of vasospasm on vascular imaging. A common side effect of nimodipine is a decrease in blood pressure, which can lead to decreased cerebral perfusion and decreased cerebral perfusion pressure. Therefore, to prevent hypotension, a dose reduction with increasing frequency to 30 mg every 2 hours may be necessary.
In all cases, reasonable maintenance of intravascular isovolemia is recommended. Decreased intravascular volume and a negative fluid balance are associated with a higher incidence of late ischemic events and poor neurological outcomes.
How to track isoquent condition is not determined. The trend of central venous manometry has broken down because it has shown a poor prediction of intravascular volume and fluid response. Measurement of pulse pressure or respiratory variation of inferior vena cava diameter using bedside ultrasonography are easy to perform and are much more reliable monitoring techniques for the fluid response of controlled patients. positive values, including SAH. Prophylactic volume expansion should be avoided, as this strategy has not been shown to improve cerebral blood flow or reduce the frequency of cerebral vasospasm or late ischemia but increase cardiopulmonary complications.
Maintenance of isotonic status can be difficult in the presence of cerebral salt loss, a common neuroendocrine disorder in SAH (see next section on hyponatremia). In patients with SAH and significant polyuria and loss of urinary sodium, the addition of fludrocortisone may be beneficial in maintaining intravascular volume and normal sodium values (fludrocortisone 0.2 mg to 0.4 mg orally every 12 months). hour).
6. Diagnosis and monitoring of late cerebral ischemia
Các chuyên gia đã khuyến cáo bệnh nhân SAH cần được chụp CT để có kết quả chính xác
Diagnosis of late cerebral ischemia is not easy. The combination of neurologic examination and imaging studies can increase early detection and appropriate treatment. Admission to a neurologic intensive care unit with regular neurological examinations by experienced nurses and physicians every 1 to 2 hours is required. Delayed cerebral ischemia should be suspected if a patient with SAH presents with a focal or diffuse neurological deficit or has a reduced Glasgow score of 2 points or more and persists for at least 1 hour and cannot be explained by clinical evidence. Other causes.
Experts have recommended that all patients with SAH undergo a head CT scan 24 to 48 hours after aneurysm treatment to identify any treatment-related infarction. Any new attenuation that is not due to EVD insertion or intraparenchymal bleeding should be considered as late ischemic cerebral infarction regardless of clinical presentation.
Patients with SAH need regular physical and imaging monitoring during the late ischemic risk period. Such monitoring is usually multidisciplinary and includes ICP measurement, cerebral perfusion pressure measurement, continuous EEG, and transcranial Doppler (TCD); DSA, CTA, and CT perfusion (CTP) are also used when indicated, as are brain tissue oxygenation monitoring and microdialysis when possible.
TCD is the earliest used and best studied of all the monitoring modalities. In the major vessels of the ring of Willis, the TCD has adequate sensitivity and specificity to detect increased cerebral blood flow secondary to cerebral vasospasm, but is highly dependent on the technician and on the skull window.
Practitioners need to be aware that TCD sensitivity/specificity is good for the middle cerebral artery and internal carotid artery but much worse for the anterior cerebral artery and circulation arteries. after. The diagnostic thresholds for cerebral vasospasm were summarized.
In addition, cerebral blood flow rates may be increased for other reasons (febrile congestion, hypertension, anemia), and therefore a diagnosis of cerebral vasospasm should be made only when The ratio of mean cerebral blood flow velocity of the intracranial vessels to the mean cerebral blood flow velocity of the extracranial internal carotid artery is high. Therefore, with the diagnosis of MCA spasm, it would be prudent to routinely measure the Lindegaard ratio (mean velocity in the middle cerebral artery/mean velocity in the ipsilateral extracranial internal carotid artery). . A Lindegaard ratio > 3 indicates cerebral vasospasm. Similar ratios apply to other major blood vessels in the skull.
DSA remains the gold standard for detecting medium and large artery spasms. CTA is now widely used and is often applied to screening for vasospasm before DSA because of its high specificity and non-invasiveness. However, CTA may overestimate cerebral vasospasm. CTP with increased mean circulating time may be of value in addition to CTA to assess cerebral hypoperfusion, but further research on the application of CTP in SAH is needed.
Brain tissue oxygen saturation, cerebral blood flow, and microdialysis monitoring can provide additional information when used in the context of multimodal monitoring and potentially detect cerebral vasospasm early before it occurs. becomes symptomatic and precedes late cerebral ischemia. Clinicians must always keep in mind the limitations of such monitoring, including being limited to monitoring local rather than whole brain regions.
Continuous scalp EEG has the advantage of tracking over large areas of the brain. Continuous quantitative EEG, where possible, can facilitate interpretation of bedside data even by personnel not trained to read EEGs. Price, and therefore less widely available, currently limits the use of continuous quantitative EEG as the standard of treatment.
It will be important to differentiate between angiographic/TCD vasospasm from clinically symptomatic vasospasm. The former occurs in the majority of patients with SAH (70%) but is not associated with post-SAH consequences. Only symptomatic vasospasm, which occurs in 30% of patients with SAH, is associated with late cerebral ischemia and poor outcome after SAH. Considering the risks of endovascular therapy for cerebral vasospasm, experts recommend such treatment only for patients with symptomatic vasospasm, whereas angiographic/TCD vasospasm should be treated. cautiously and wait with a very low threshold for DSA activation and endovascular therapy.
There are several different views on the timing and frequency of adopting different tracking methods. At the very least, care for patients with SAH should be regimented using a handwritten protocol and an algorithm. Patients with SAH should be admitted to the neuro-intensive care unit and have their aneurysms sealed as soon as possible, preferably within the first 6 to 12 hours of admission.
Neuro-intensive unit monitoring includes daily TCD, although in low-risk patients it may be sufficient to monitor every other day as long as clinical neurologic monitoring can be performed every 1 up to 2 o'clock. Some centers perform routine CTA/CTP or DSA on all patients 5 to 7 days after admission to screen for cerebral vasospasm. Most centers, however, perform these imaging tests only when symptomatic vasospasm is suspected.
In comatose or unresponsive (inert) patients, a reliable clinical examination may be difficult to perform, and therefore TCD and, in addition, CTA/CTP or DSA to screen for vasospasm may be necessary . Especially in patients with severe SAH with severe neurological symptoms, late diagnosis and initiation of treatment for ischemic stroke can be difficult, slightly perceptual, and largely dependent on imaging findings.
At the author's hospital, the management regimen for SAH consisted of inducing hypertension, CT/CTA, and DSA when these patients had an increase in mean TCD rate and an increase in the Lindegaard ratio (or other ratio). Other centers use information from multimodality monitoring (continuous EEG or even deep cortical EEG, cerebral tissue oxygen saturation, microdialysis, cerebral blood flow monitoring) to determine whether hypoperfusion is present. brain vasospasm or not and when to do a DSA. While randomized controlled data are lacking on the value of multimodality monitoring for short- and long-term outcomes in the treatment of patients with SAH, multi-hospital resources may lead us to, at least to some extent, apply a variety of advanced multimodal monitoring measures.
7. Complications Management
At the author's hospital, all patients were treated with nimodipine and isovol. Low-risk patients with neurologic examination, TCD and, if performed, unchanged CTA should be referred to a milder neurology unit under the care of a neurologist between days 8 and 10. and received less frequent neurological examinations (every 2 hours or every hour) than in the ICU. The patient is then discharged home on day 14. High-risk patients with neurologic examination, TCD, and, if performed, CTA remaining unchanged will be referred to the home or light unit. other 14 days after SAH. If at any time an increase in mean cerebral blood flow velocity on the TCD and an abnormal CTA is observed, the intensity of neuromonitoring should be increased.
If the patient's neurologic deterioration suggests late cerebral ischemia, some rescue therapy should be initiated. In cases of cerebral vasospasm and symptomatic late cerebral ischemia, hypertension is indicated according to current guidelines. Volume, hypertension, and hemodilution (Triple H) therapy is currently not supported by guidelines because of the available evidence for adverse effects following hemodilution, and Current standard treatment is hypertension and mild hypervolemia (HHT). Many hospitals start with a bolus of intravenous fluid (1 L to 2 L of 0.9% saline) and maintain fluid to isovolemic or slightly hypervolemic. Hypertension is best induced using a continuous intravenous infusion of α1 receptor agonists (norepinephrine or phenylephrine). This class of drugs is the vasoconstrictor of choice in SAH, because cerebral blood vessels lack the α1 receptor, and thus are only systemic vasoconstrictor but not cerebral vasoconstrictor. Hypertension should be performed incrementally with periodic neurological evaluation at each step.
At the author's hospital, mean arterial pressure (MAP) above baseline MAP of 20 mmHg was set for the first goal (usually MAP > 90 mmHg). Some facilities use systolic blood pressure targets instead of MAP targets, and there is currently no evidence to guide clinicians as to which metric is better. In settings where a systolic blood pressure target is set for inducing hypertension, the baseline should be approximately 20 mmHg to 40 mmHg above baseline systolic pressure. This often results in a systolic blood pressure target >180 mmHg or >200 mmHg.
If physical examination returns to baseline, no further BP elevation is necessary, unless clinical examination is more severe at baseline. In the latter case, further elevation of the blood pressure target should be attempted. Although there is no maximal or optimal blood pressure target, cardiac and pulmonary and cerebral adverse events (eg, autoregulatory side effects with elevated ICP due to elevated MAP or reversible posterior encephalopathy syndrome) [PRES]) should be considered in each patient.
At the author's hospital, cardiotropic agents (milrinone, dobutamine) were reserved for patients with known low cardiac output due to acute or chronic cardiomyopathy. If neurologic deficits persist despite hypertension, the patient should undergo CT/CTA followed by DSA with endovascular therapy if cerebral vasospasm is identified. Non-contrast head CT prior to DSA is useful to rule out hydrocephalus and identify previous stroke prior to endovascular therapy. If other causes of neurologic deterioration have been ruled out, and the TCD suggests cerebral vasospasm, the CTA may be omitted to limit the patient's radiation exposure, iodinated contrast, and to save time. and allow the patient to go for a DSA immediately for treatment. Endovascular therapy using intra-arterial vasodilators (nicardipine, milrinone, verapamil) or angioplasty is supported by prospective and retrospective observational data and recommended by guidelines. In the author's hospital, prophylactic angiography was not performed if cerebral vasospasm was detected during TCD or CTA without neurological deterioration because this practice was associated with a high complication rate.
Source: Susanne Muehlschlegel. Subarachnoid hemorrhage. CONTINUUM (MINNEAP MINN) 2018;24(6, NEUROCRITICAL CARE): 1623–1657