This is an automatically translated article.
Post by Master, Doctor Mai Vien Phuong - Gastrointestinal Endoscopy - Department of Medical Examination & Internal Medicine - Vinmec Central Park International General Hospital.
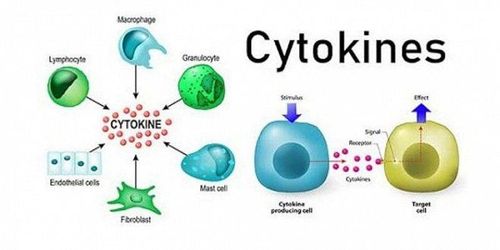
Hepatitis b virus (HBVr) reactivation may occur in patients treated with immunosuppressive therapy and chemotherapy. Therefore, in the current biological age, it is important for physicians to understand the risk of hepatitis B virus reactivation in patients with autoimmune disease undergoing anti-cytokine therapy.
1. Overview
The following three components are important for the development of hepatitis B virus reactivation:
The host immune response; Covalent closed circular DNA of the HBV viral genome (cccDNA); The use of immunosuppressive drugs. HBV infection induces a variety of innate and adaptive immune responses. The host immune responses against hepatitis B virus (HBV) infection allow adaptive cytotoxic T (Tc) cells to produce both mitotic-dependent, antiviral effects. and independent of the indication.
In its mitotic-independent effect, interferon (IFN) plays an important role to suppress HBV replication. To induce circulating HBV-neutralizing antibodies, B cells are also recruited to limit the spread of HBV virus. However, even if clinical analysis of HBV infection is obtained, it does not mean complete eradication of HBV-DNA because cccDNA may persist in the nuclei of hepatocytes and it may be a source of Hepatitis B virus reactivation with immunosuppressive drugs.
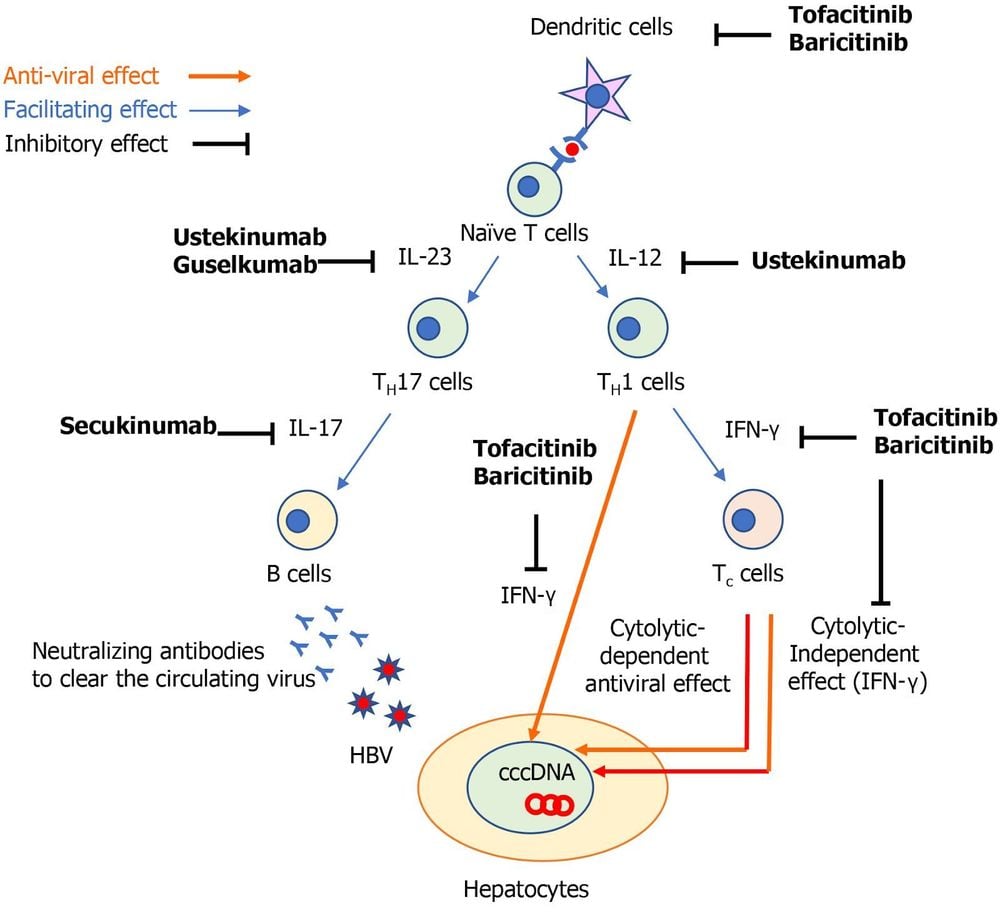
2. The role of tumor necrosis factor (TNF) -α
Tumor necrosis factor (TNF)-α is an important cytokine not only in the pathogenesis of autoimmune diseases but also in host immune responses against HBV infection. TNF-α is synthesized by macrophages and T cells and produces a variety of inflammatory cytokines, which block viral replication. TNF-α is also required for the proliferation of HBV-specific Tc cells required to inhibit HBV replication. Therefore, TNF-α inhibitors (eg, infliximab, adalimumab, and etanercept) can suppress the anti-HBV immune response, leading to HBV replication. The overall prevalence of hepatitis B virus reactivation in patients with autoimmune diseases receiving TNF-α inhibitors has been reported to be 4.2% (95% CI: 1.4% - 8.2%).
The signaling pathways involving interleukin (IL)-12/23, IL-17 and Janus kinases (JAKs) have been marked as novel specific therapeutic targets for autoimmune diseases. A recent multicenter observational study for psoriasis found that hepatitis B virus reactivation was more common in patients treated with anti-TNF-α drugs than with IL-17 inhibitors. . However, there are still limited data in understanding the risk of hepatitis B virus reactivation in patients treated with biologics that inhibit such specific inflammatory pathways.
3. HBV infection and response
Professional associations in the United States [American Association for the Study of Liver Disease (AASLD); The American Gastroenterology Association (AGA)], Europe [European Association for the Study of the Liver (EASL)] and Asia [Asia Pacific Association for Liver Research (APASL)] have published guidelines to assist providers in managing hepatitis B virus reactivation. Specifically:
chronic HBV: chronic HBV (CHB) [ie, hepatitis B surface antigen (HBsAg) - positive and antibody to hepatitis B core antigen (anti-HBc) - positive] included patients who were chronically active [HBV DNA serum 2000 IU/mL and normal or elevated serum alanine transaminase (ALT)] or inactive (HBV DNA <2000 IU/mL and HIV infection). HBV ALT normal). HBV has recovered: HBV has recovered (i.e. HBsAg negative and anti-HBc positive). Of note, there is insufficient evidence to support the use of anti-HBs titres as a decision support measure when making recommendations for prophylaxis. There are slight differences in the definition of hepatitis B virus reactivation between guidelines, but the general concept is the same. In chronic HBV patients, hepatitis B virus reactivation is defined by an increase in HBV DNA above baseline. In patients with resolved HBV, hepatitis B virus reactivation is determined by the appearance of HBV DNA in the blood or transition to the HBsAg+ state (ie, seroconversion).
The observed heterogeneity in the definition of hepatitis B virus reactivation is also reflected in the available studies on hepatitis B virus reactivation. Most studies include the following parameters:
The level of HBV-DNA increases rapidly from baseline; Increased serum aminotransferase levels; Serum reversal.
4. Management of hepatitis b virus patients on immunotherapy
4.1. Risk hierarchy Patients with chronic HBV are at increased risk for hepatitis B virus reactivation during immunosuppressive therapy compared with patients with resolved HBV. For example, among patients treated with TNF-α inhibitors, there is an estimated 5-fold increased risk of hepatitis B virus reactivation in chronic HBV patients compared with recovered HBV patients. recovery (15.4% vs 3.0% risk of hepatitis B virus reactivation).
Another study showed that the composite rate of hepatitis B virus reactivation without antiretroviral prophylaxis was 15.6% (95% CI: 2.3-35.7) in patients with hepatitis B virus. Chronic HBV patients are treated with TNF-α inhibitors. In patients with resolved HBV, the pooled rate of hepatitis B virus reactivation without antiretroviral prophylaxis in patients treated with TNF-α inhibitors was also as biologic treatment does not target TNF of 1.4% (95% CI: 0.5%-2.6%) and 6.1% (95% CI: 0.0%-16.6%) , corresponding. Next, the degree of iron-induced immunosuppression should be assessed. Recipients of hematopoietic stem cell transplantation (HSCT) and B-cell-depleting therapies (eg, rituximab) are the most potent regimens and have the highest risk of hepatitis B virus reactivation. The AGA guidelines state that anthracyclines (eg, doxorubicin) and moderate to high dose corticosteroids (CS) (ie, ≥ 10 mg of prednisone daily or equivalent for ≥ 4 weeks) pose a higher risk than with other immunosuppressive drugs.
4.2 . Chronic HBV Prophylaxis In general, occupational and social guidelines recommend HBV prophylaxis, typically entecavir or tenofovir, for all immunosuppressive candidates with chronic HBV, with the exception of those patients treated with traditional immunosuppressants (eg, thiopurine, methotrexate), intra-articular CS, or oral CS for ≤ 1 week. The AGA stratifies this patient population into moderate (1%-10%) and high (>10%) risk for hepatitis B virus reactivation. Antiretroviral prophylaxis should be initiated. before and continue after stopping immunosuppression, usually 12 to 18 months if high-potency therapies are used and 6 to 12 months for other therapies.
HBV recovered The guidelines largely agree that HBV patients who have recovered with high-potency immunosuppressive therapy (recipients of HSCT and B-cell-depleting therapy) should receive prophylactic treatment of the disease. hepatitis B virus reactivation. AGA classifies this patient group as a high-risk (>10%) group of hepatitis B virus reactivation. For recovered HBV patients not on high-potency regimens, the guidelines are more different. The AGA recommends prophylaxis for recovered HBV patients at moderate risk (1%-10%) of hepatitis B virus reactivation, including patients treated with TNF-α inhibitors. , other cytokines or integral inhibitors, tyrosine kinase, medium or high dose CS for ≥ 4 wk as well as anthracycline derivatives.
In contrast, AASLD, EASL, and APASL recommend a preferred therapy for this group of patients, not prophylaxis. Accordingly, serial laboratory monitoring (HBV DNA, HBsAg) was performed at intervals of 1 to 3 months during treatment and up to 12 months after cessation of immunosuppression with antiretroviral therapy as required. request if needed. Because HBsAg seroconversion can lead to fatal acute hepatitis, antiviral therapy should be initiated immediately, independent of ALT levels. Of note, both EASL and APASL recommend the same treatment of patients with recovered HBV as HBsAg-positive patients if baseline serum HBV-DNA is positive. The AGA classifies recovered HBV patients treated with traditional immunosuppressants (eg, thiopurines, methotrexate), low-dose CS for ≥4 weeks, intra-articular CS, or any oral dose of CS for ≤1 week, is a low risk (<1%) for hepatitis B virus reactivation and does not recommend prophylaxis, similar to other societal guidelines.
5. Risk of hepatitis B virus reactivation in patients on non-TNF-targeted biologic therapy
Because of limited data on the risk of hepatitis B virus reactivation in patients with non-TNF-targeted biologics, the authors reviewed the available literature on the risk of hepatitis B virus reactivation. B in patients with autoimmune disease who have received biologics that do not target TNF. The majority of articles focused on patients with chronic HBV or resolved infection. According to the AGA guidelines, patients with chronic HBV and recovered HBV treated with non-TNF-targeted therapies are classified as intermediate-risk hepatitis B virus reactivation and should be treated. preventive.
However, AASLD, EASL and APASL recommend serial monitoring between recovered HBV (if HBV DNA is negative) with prior prophylaxis if hepatitis B virus reactivation is observed. It is important to accurately determine hepatitis B virus reactivation by biological products that do not target TNF.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References: Akiyama S, Cotter TG, Sakuraba A. Risk of hepatitis B virus reactivation in patients with autoimmune diseases undergoing non-tumor necrosis factor-targeted biologics. World J Gastroenterol 2021; 27(19): 2312-2324 [DOI: 10.3748/wjg.v27.i19.2312]