This is an automatically translated article.
Pandemic of the H3N2 influenza virus was first recorded in the United States in September 1968. The estimated death toll of the pandemic is 1 million worldwide and approximately 100,000 in the United States. Most deaths from the H3N2 virus that cause illness are mainly in people 65 years of age and older.
1. Learn about the H3N2 . Virus strain
Influenza virus (H3N2) consists of two genes from influenza A virus, hemagglutinin H3 and N2 neuraminidase from the 1957 H2N2 virus. Its name is therefore also derived from forms of two surface proteins hemagglutinin (H) and neuraminidase (N). By recombination, the H3N2 virus exchanges genes for internal proteins with other influenza subtypes.
H3N2 virus can infect birds and mammals. In birds, humans and pigs, the virus has mutated into multiple strains. During the years when the H3N2 virus was the predominant strain, there were many hospitalizations and possibly even death.
The H3N2 influenza virus continues to circulate worldwide as a seasonal influenza A virus. Seasonal influenza A viruses are associated with severe illness in older adults, and experience frequent antigenic drift.
Virus cúm H3N2
2. Variation of influenza virus
Flu viruses are constantly mutating and they can change in two ways:
2.1. Antigen drift Antigen drift are small changes (or mutations) in influenza virus genes that can lead to changes in the surface of the HA (hemagglutinin) and NA (neuraminidase) viruses. The influenza virus HA and NA surface proteins are antigens, which means that they are recognized by the immune system and have the ability to trigger an immune response (including the production of antibodies that can block infection). ). These changes are related to antigenic drift, and occur continuously over time as the virus replicates. Most flu shots are designed to target a virus or its surface antigen.
Small changes resulting from antigenic drift often produce viruses that are closely related, and can be illustrated by their proximity to each other on the phylogenetic sequence. Closely related influenza viruses often have similar antigenic properties. This means that the antibodies the immune system produces against a virus that are able to recognize and respond to influenza viruses are antigenically similar (super-cross-protection).
However, small changes associated with antigenic drift can accumulate over time and lead to antigenically different viruses. It is also possible that a small change in a particularly important site on the BP leads to antigenic drift. With antigenic drift, the body's immune system may not recognize and prevent illness caused by newer influenza viruses. As a result, a person who has been infected with the flu can get a relapse because of antigenic drift that changes the virus.
Antigen drift makes it possible for people to get the flu more than once and is why flu vaccine components must be reviewed and updated each year to keep up with the evolution of the virus.

Tiêm vắc-xin phòng cúm H3N2
2.2 Antigen Shift Antigen shift is a sudden, mainly antigenic shift of influenza A virus that results in a new virus or new HA and NA proteins in infectious human influenza viruses. This change can occur when influenza viruses from animal populations are able to infect humans. Such viruses of animal origin may contain a different HA or HA/NA combination than that found in humans to which most people do not have immunity to the new virus.
Such a change occurred in the spring of 2009, when an influenza virus with genes from North American pigs, Eurasian pigs, humans and birds emerged to infect humans and rapidly spread causing a pandemic. Translate. When the change occurs, most people have little or no immunity against the new virus.
While viruses are constantly changing due to antigenic drift, antigenic shifts are less frequent. Pandemic influenza is also rare. In the last 100 years there have been four such pandemics.
See the following article to understand more about this issue: Why do influenza virus strains change so often?
3. H3N2 flu pandemic in 1968
In 1957, a new influenza pandemic appeared in Southeast Asia. Once again, the daily press sounded the alarm with a brief report on the major epidemic in Hong Kong in the Times of London.
As this epidemic progressed, initially across Asia, important differences in morbidity and mortality patterns were noted. In Japan, the epidemic was small and sporadic until late 1968. Most notably, the high morbidity and mortality rates in the United States after the introduction of the virus on the West Coast.
Because the Hong Kong virus differs from the previous Asian virus by its HA antigen, but it retains the NA (N2), NA (N16) antigens. The researchers speculated that its variable effects and sporadic transmission in different regions of the world were mediated by differences in prior N2 immunity.
Thus, the pandemic of 1969 was characterized as "smoldering". Further evidence on the ability of previous N2 experiences to moderate the Hong Kong virus challenge is provided by Eickhoff and Meiklejohn. Two scientists showed that vaccinating air force cadets with an H2N2 adjuvant vaccine reduced influenza after infection with the H3N2 virus.
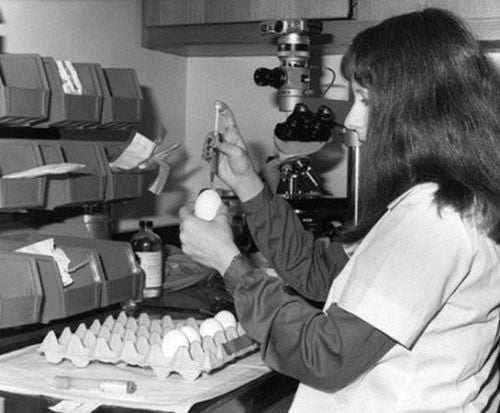
Một nhân viên thí nghiệm đang tiêm virus cúm Hong Kong vào trứng gà (năm 1968)
The improvement of H3N2 virus infection by NA immunity alone is most notable because of its virulence as occurred in 1918 and 1957. Although, not necessarily indicative of virulence, but interspecies transmission of the virus has been observed. Thirty-seven years later, the H3N2 subtype still reigns as the main and most troublesome human influenza A virus.
References: cdc.gov; ncbi.nlm.nih.gov
Recommended video:
Livestream: How to prevent and treat flu effectively
MORE:
How long does the seasonal flu usually last? All you need to know about seasonal flu Influenza: When to go to the hospital?