This is an automatically translated article.
The article is made by Doctor - Laboratory Doctor - Microbiology - Laboratory Department - Vinmec Danang International General Hospital.
Mother-to-child transmission of hepatitis B virus is the main long-term transmission route in areas where the prevalence of HBV - hepatitis B virus is over 8% of the population. If not vaccinated from birth, 90% of babies born to mothers with the HBV virus will become hepatitis B carriers for life.
Hepatitis B virus is the leading cause of chronic hepatitis , cirrhosis , and especially liver cancer . Hepatitis B virus infection is a global social problem. About one third of the world's population (more than 2 billion people) has a history of hepatitis B virus infection, and currently there are about 400 million chronic hepatitis B virus carriers in the world. One of the regions with the highest prevalence of hepatitis B virus infection in the world and also home to the highest number of chronic hepatitis B virus patients is the Western Pacific region (including China, Vietnam and other countries). other Southeast Asian countries). If not vaccinated at birth, 90% of babies born to mothers infected with the hepatitis B virus will become hepatitis B virus carriers for life. In which, about 25% of these babies will die of cirrhosis or liver cancer later on. Counseling and preventive treatment to limit the transmission of hepatitis B virus from pregnant women to their children is of great significance in order to reduce the prevalence of hepatitis B virus infection in the community, and at the same time contribute to the generation of future generations. grow up healthy.
Virus viêm gan B có nguy cơ lây nhiễm từ mẹ sang con
1. Immune Tolerance Phase
This phase lasts from 10-30 years. During this phase:
HBeAg (+). Does not cause hepatitis with normal ALT/AST liver enzymes, minimal histological damage to the liver, no evidence of active hepatitis, and no clinical symptoms. HBV replicates very strongly with very high serum HBV-DNA levels due to the body's immune tolerance to HBV. The immune system does not prevent HBV from multiplying. Immune tolerance is considered to be the main cause of HBeAg (+) and normal liver enzymes. This is a process that inhibits the immune response of T lymphocytes to HBeAg and HBcAg. Leads to ineffective T-lymphocyte-mediated destruction of HBV-infected hepatocytes.
Ở giai đoạn dung nạp miễn dịch, bệnh nhân nhiễm HBV vào thời kỳ sơ sinh có chỉ số HBeAg (+)
2. Immune Clearance or chronic hepatitis HBeAg (+)
During this stage there is an immune response of the body to HBV. The immune system in the child's body has matured and recognizes the liver cells infected with HBV and begins to attack these cells, causing HBeAg (+) chronic hepatitis B. HBV still duplicates but reflects clearance of HBV-DNA because HBV-DNA levels decrease compared with the immune tolerant phase. Accompanied by a biochemical exacerbation, the liver enzymes ALT/AST are very elevated due to the sudden immune-mediated destruction of infected liver cells by T lymphocytes. Usually 50-70 % of patients increased HBeAg elimination and seroconversion from HBeAg (+) to HBeAg (-) at the end of this period. Most exacerbations during the immune clearance phase are asymptomatic and detected during follow-up. Some have symptoms such as acute hepatitis and the appearance of anti-IgM, so they are misdiagnosed with acute hepatitis B.
Not all exacerbations during the immune clearance phase lead to HBeAg seroconversion and HBV-DNA clearance. These patients may have recurrent exacerbations with intermittent HBV-DNA disappearance and with or without transient HBeAg disappearance. Such recurrent exacerbations increase the risk of cirrhosis and liver cancer. In particular, a very small number of exacerbations during the immune clearance phase lead to decompensated liver failure and possibly death.
Suy gan có thể xảy ra trong các đợt kịch phát trong giai đoạn thải trừ miễn dịch
3. Non-replicating virus phase or inactive carrier phase (Inactive carrier)
During this phase, HBeAg is negative, anti-HBe is positive, serum HBV-DNA is low <104copies/ml or undetectable, liver disease is in remission, ALT/AST expression is not elevated, and liver biopsy shows decreased levels. caseation. Approximately 0.1-0.8 % of Asians lose HBsAg each year. Some patients may go through a phase of reactivation after a period of time. This stage can last a lifetime.4. Reactivation or chronic hepatitis HBeAg (-) precore mutation
Approximately one-quarter of Asian patients with chronic hepatitis B have HBeAg (-) after HBeAg seroconversion.
HBV has a precore mutation HBeAg (-) that allows HBV to duplicate again and suppresses the immune system, causing chronic hepatitis B with indicators such as HBV - DNA reappearing in the serum, liver enzymes ALT/AST increased again, HBeAg (-) and anti HBe (+).
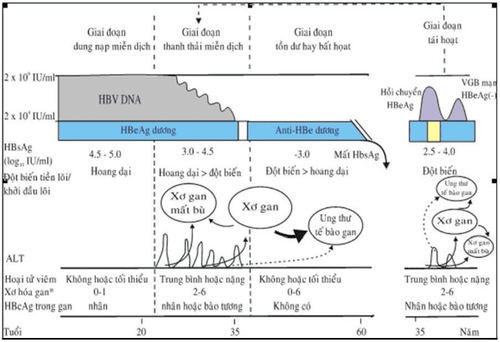
Hình 1. Lịch sử tự nhiên của nhiễm VGB mạn mắc phải chu sinh và trong thời kỳ thơ ấu
5. Factors that adversely affect the progression of chronic hepatitis B
Host factor :
Age > 40 Male Immune status. Viral factors:
HBV concentration - high DNA HBV genotype C Slow HBeAg seroconversion. Environmental factors:
Alcohol consumption Diabetes obesity Co-infection with viruses C, D.

Uống rượu bia làm ảnh hưởng xấu đến diễn tiến của viêm gan B mạn
6. When to treat patients with HBV infection in infancy
TREATMENT ONLY WHEN THE PATIENT IS IN THE Immune CLEARANCE OR REACTIVE STAGE.6.1. Identify cases that do not require treatment
When the virus in the patient's body is inactive, so no drug treatment is needed. Determined when:
When HBsAg (+) but HBeAg (-) Hepatitis B virus quantification: HBV DNA result above or below 10 4 copies/ml Liver enzyme index ALT/AST less than 40 UI/ml Ultrasound The liver was not necrotic.
6.2. Identify cases requiring drug treatment
When the virus is multiplying, it is necessary to take medicine immediately:
When HBsAg (+) and HBeAg (+) Hepatitis B virus quantification: HBV-DNA result above 10 5 copies/ml Liver enzyme index increases more than 2 times Normal Ultrasound shows liver necrosis Accompanied by clinical symptoms of fatigue, anorexia, jaundice, yellow eyes, right lower quadrant pain.....

Khi virus đang nhân lên và kèm theo triêu chứng vàng da cần điều trị bằng thuốc
6.3 Importance of quantitative testing of hepatitis B virus HBV - DNA
Based on the results of PCR analysis to measure the hepatitis B virus load with HBV-DNA technique, the patient can understand his own health situation and the doctor will have a basis to determine which cases are not necessary. medication or need to use antiviral drugs.
However, the HBV-DNA technique to measure hepatitis B viral load by PCR can still be erroneous under conditions of improper sampling and storage and the procedure is not of professional quality, so It can make it difficult for clinicians to accurately analyze the patient's disease to inform treatment decisions.
Vinmec International General Hospital is one of the hospitals that not only ensures professional quality with a team of leading medical doctors, modern equipment and technology, but also stands out for its examination and consultation services. comprehensive and professional medical consultation and treatment; maximum safety and sterilization space. Customers when choosing to perform tests here can be completely assured of the accuracy of test results.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
REFERENCES
Infectious and Tropical Pathology - Department of Infectious Diseases - Medical Publishing House - Hanoi - 2008 Hepatitis B Virus - Nguyen Van Mui - Medicine Publishing House - Hanoi - 2002 Anna SF Lok, Rafael Esteban, Peter A L Bonis. Clinical manifestations and natural history of hepatitis B virus infection. Up to date version 17.1: January 2009. 4 Lok ASF, McMahon BJ. Hepatology. 2009;50:661-662 5. Tsang, TK, Blei, AT, O'Reilly, DJ, Decker, R. Clinical significance of concurrent hepatitis B surface antigen and antibody positivity. Dig Dis Sci 1986; 31:620. 6. Yeo W, Chan PK, Zhong S, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol 2000;62(3):299-307.