This is an automatically translated article.
Mifepristone is an active ingredient used extensively in obstetrics for the purpose of terminating pregnancy or inducing labor in stillbirth. This active ingredient is in the product Mife 200. So what is Mife 200 and how should it be used?
1. What is Mife 200?
Mife 200 contains the active ingredient Mifepristone 200mg, in the form of tablets and is indicated in the termination of pregnancy with a gestational age of less than 22 weeks or induction of labor in case of stillbirth. Mife 200 is a product of Hera Biopharmaceutical Co., Ltd (Vietnam).
2. Pharmacology of the drug Mife 200
The active ingredient Mifepristone in Mife 200 acts as a Progesterone antagonist by competitively binding to endogenous Progesterone receptors. Mife 200 has a very high binding affinity for these receptors, up to 2-10 times that of Progesterone.Due to the inhibition of receptor binding, Mife 200 blocks the effects of progesterone on the endometrium and peritoneum, leading to mucosal degeneration and shedding and consequently helps prevent or disrupt binding. tightening of the fetus on the lining of the uterus.
Pharmacokinetics of Mife 200:
Bioavailability of Mife 200 is about 70% when used orally. Peak plasma concentrations of mifepristone are achieved 1 to 2 hours after a single oral dose; The half-life of Mife 200 is about 20 to 30 hours; Unbound mifepristone is rapidly metabolised via Di-methylation and Hydroxylation in the liver. Notably, this metabolism can be observed in plasma about 1 hour after oral administration; Mife 200 and its metabolites are eliminated mainly in the faeces via biliary excretion and to some extent by renal excretion.
3. Indications and contraindications of Mife 200
Mife 200 products are indicated for use in the following cases:
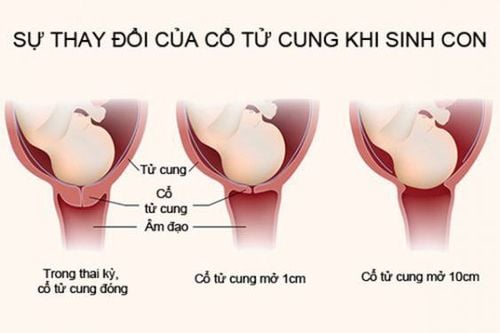
Termination of the development of pregnancy in the uterus until the end of the 22nd week of pregnancy; Induction of labor in women diagnosed with stillbirth. Cases of contraindications to the use of Mife 200:
People who are allergic to Mifepristone, Misoprostol or any of the ingredients in Mife 200; The patient is identified with adrenal insufficiency; Patients with severe asthma who have not been adequately controlled on treatment; People with inherited disorders of porphyrin metabolism; Patients with mitral valve, embolism or history of embolism; People with blood clotting disorders or being treated with anticoagulants; Moderate to severe anemia. In case of need to terminate pregnancy from 9-10 weeks gestation to the end of 12 weeks, absolutely do not use Mife 200 when:
Suspected or confirmed ectopic pregnancy; The fetus clings to the old surgical scar in the uterus. With indications for termination of pregnancy from the 13th to the 22nd week, Mife 200 is absolutely contraindicated when:
Pregnant women have old surgical scars in the body of the uterus; Comb braces.
4. How to use Mife 200
4.1. Dosage of Mife 200 in terminating pregnancy In case of gestational age 63 days:
Pregnant women take 1 tablet of Mife 200; After 24 to 48 hours, continue to sublingual or buccal 800 mcg Misoprostol at the hospital or at home depending on the gestational age and the mother's wishes; Pregnant women from the 8th to the 9th week should use Mife 200 and monitor miscarriage at the hospital; The doctor will choose a convenient time to use Misoprostol for pregnant women. Gestational age from 64 to the end of 84 days:
Start with 1 tablet of Mife 200; After about 24 to 48 hours, carry out intravaginal insertion of 800 mcg Misoprostol at the hospital; Continue monitoring every 3 hours with 400 mcg of Misoprostol sublingually, up to 4 times until complete abortion; If, after 3 hours from the time of taking the 5th dose of Misoprostol, there is still no miscarriage, then give the woman another dose of Mife 200, then give the woman a break for 9-11 hours and then repeat the Misoprostol doses as above until the pregnancy is over. miscarriage; If following the above regimen twice and still no miscarriage, the doctor may consider switching to another method. Gestational age from 13 to 18 weeks:
Initially, women take 200mg Mifepristone (equivalent to 1 Mife 200 tablet); After 24-48 hours, carry out intravaginal insertion of 400 mcg of Misoprostol and every 3 hours for the mother to sublingual or buccal 400 mcg of Misoprostol until miscarriage; If after 5 doses of Misoprostol there is still no miscarriage, the next day, take another 5 doses of 400mcg sublingually every 3 hours until miscarriage; In case the pregnancy still does not abort, the woman should continue to use Mife 200 for the 3rd day according to the above regimen. If after 3 days still no miscarriage, use another method. Gestational age from 19 to 22 weeks:
Start giving women 1 tablet of Mife 200; After 24 - 48 hours of intravaginal insertion of 400mcg misoprostol, then every 3 hours sublingually 400mcg Misoprostol until miscarriage; After 5 doses of Misoprostol as above without miscarriage, the next day take another 5 doses of 400mcg Misoprostol sublingually every 3 hours until miscarriage. If the pregnancy still does not miscarry, switch to another method. 4.2. Promote stillbirth For the patient to use 3 Mife 200 tablets per day, for 2 consecutive days.
Note that in all cases, the use of Mife 200 always requires measures to block the Rh sensitization factor (if the patient is Rh negative) along with some general measures throughout the duration of the test. termination of pregnancy.
The patient can become pregnant again as soon as the termination of pregnancy is over. However, because Mife 200 still carries a risk of effects, it is recommended that patients avoid becoming pregnant again before having their next menstrual period after taking Mife 200.
5. Note when using Mife 200
In some cases, treatment with Mife 200 may not be suitable, so patients should inform their doctor if they have the following problems:
History of cardiovascular diseases; Have cardiovascular risk factors, such as high blood pressure or high blood cholesterol levels; History of bronchial asthma ; Have diseases that affect blood clotting; History of liver or kidney disease; Patients with anemia or malnutrition. Vaginal bleeding when using Mife 200 may be prolonged or worse (on average about 12 days after taking Mife 200). It should be noted that this vaginal bleeding is not related to the success of the treatment with this preparation.
Use of Mife 200 for pregnant women:
In clinical practice, it is very rare to have lower extremities deformities after using Mife 200 as monotherapy or in combination with Prostaglandins; Cases of unsuccessful termination of pregnancy with Mife 200, the risk of negative effects on the fetus is unknown; If treatment with Mife 200 fails and is diagnosed at routine checkups (and if you agree), your doctor may recommend another method of termination of pregnancy; If a woman wishes to continue becoming pregnant after a failed attempt at taking Mife 200, her doctor should explain the possible risks to the fetus. Then the mother should be examined by fetal ultrasound carefully before giving birth, with special attention to the baby's limbs; After taking Mife 200, women should not get pregnant again before the next menstrual period. Use of Mife 200 in lactating women:
Because Mife 200 can pass into breast milk, mothers need to stop breastfeeding during treatment with this preparation. Mife 200 does not affect the ability to drive and use machines. If a headache occurs, be careful when driving or operating machinery.
6. Side effects of the drug Mife 200
Some very common side effects of Mife 200 :
Uterine contractions or cramps a few hours after taking the drug; Some of the effects associated with the use of Prostaglandins include nausea, vomiting, or diarrhea. Some common side effects when using Mife 200:
Heavy bleeding; Post-abortion infection; Mild to moderate gastrointestinal spasms. In addition, Mife 200 may cause some uncommon side effects such as skin rash, low blood pressure, headache, malaise, vagus nerve symptoms (such as hot flashes, dizziness, chills). , fever and sometimes serious skin disorders (exfoliative dermatitis, epidermal necrolysis and erythema).
7. Drug interactions of Mife 200
Mife 200 should not be used concurrently with Aspirin and other NSAIDs, because inhibitors of Prostaglandin synthesis could theoretically alter the effect of Mifepristone.
Mifepristone is an active ingredient used extensively in obstetrics for the purpose of terminating pregnancy or inducing labor in stillbirth. Using the drug exactly as directed by the doctor will bring high efficiency and avoid unwanted side effects.
Follow Vinmec International General Hospital website to get more health, nutrition and beauty information to protect the health of yourself and your loved ones in your family.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.