This is an automatically translated article.
Posted by Master, Doctor Mai Vien Phuong - Department of Examination & Internal Medicine - Vinmec Central Park International General Hospital
Dysbiosis is a term that includes a change in the composition of the combined microbiota compared with that found in healthy individuals. When differentiating the biome of a normal healthy esophagus from dysbiotic disease states such as reflux esophagitis, esinophilic esophagitis, Barrett's esophagus, or adenocarcinoma of the esophagus , it is clear that dysbiosis may precede inflammation.
Therefore, the composition of the microbiome may play a large role in downstream events. Gram-negative bacterial products activate Toll-like-receptors present on esophageal endothelial cells causing an inflammatory cascade that leads to relaxation of the lower esophageal sphincter. Prevotella, found more commonly in the distal esophagus with dysbiosis, has been shown to be a major producer of lipopolysaccharides that contribute to Toll-like-receptor activation. Activation results in the chemokine-induced production of nitric oxide and COX-2, which may promote relaxation of the lower esophageal sphincter and decrease gastric emptying.
Microbial imbalance in upper gastrointestinal diseases
Reflux esophagitis Reflux esophagitis is an inflammatory disease state usually caused by inadequate transient relaxation or secondarily by chronic hypotonicity of the lower esophageal sphincter. It is further classified into two major phenotypes: RE and NERD, identified by endoscopy. In addition, there is an untreated type of reflux esophagitis in which treatment is initiated without guidance from endoscopic evaluation.
Inflammatory pathogenesis of reflux esophagitis:
Classically, reflux esophagitis has been described as the result of reflux of gastric acid and/or duodenal bile salts, causing damage and direct chemically mediated mucositis. The majority of patients with clinical symptoms of reflux esophagitis have no endoscopic evidence of reflux, suggesting alternative pathophysiological pathways.
An animal study demonstrated that RE does not develop as a chemical lesion that begins at the epithelial surface but begins with submucosal infiltration by lymphocytes which then progresses to the surface. epithelial surface. Subsequent work analyzed human esophageal squamous cell lines exposed to acidified bile salts and found evidence that reflux does not directly damage the lining of the esophagus, but instead irritates it. epithelial cells and leads to mucosal injury upstream and mediated by subepithelial neoplasia.
Acid exposure is thought to contribute to mucosal expression of inflammatory markers, including IL-8, as well as several other chemokines, promoting local migration of leukocytes, mainly leukocytes neutral. This is accomplished through the activation of the transient receptor potential cation channel V member 1 on epithelial cells and neurons, resulting in calcitonin gene-related peptide (CGRP) and P expression. Both CGRP and neutrophilactivation initiate a cascade of cytokine expression, leading to localized submucosal inflammation as well as production of hydrogen peroxide as well as other immune cell production, including lymphocyte proliferation, and mucosa. Peroxide-mediated smooth muscle relaxation of the lower esophageal sphincter further contributes to reflux.
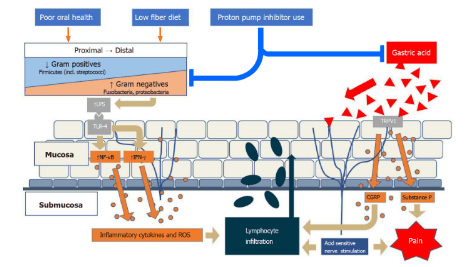
Environmental factors alter the local esophageal microbiota, often with a gradient from gram-positive to gram-negative, in the direction of an increased gram-negative ratio. Activation of Toll-like-receptor-4 by gram-negative lipopolysaccharide leads to an inflammatory cascade leading to lymphocyte infiltration. In addition, gastric acid activates the transient receptor potential cation channel V member 1 on local nerve fibers and results in calcitonin and substance P gene-associated peptide expression, which contributes to this response. local inflammation as well as pain. lipopolysaccharide : Lipopolysaccharide; Toll-like-receptor : Toll-like-receptor; NF-κB: nuclear factor-kappa beta; IFN: Interferon; ROS: Reactive Oxygen Types; TRPV1: Transient receptor potential cation channel V member 1; CGRP: Calcitonin gene-related peptide. Role of the microbiome in reflux esophagitis:
Gram-negative bacterial products, mainly lipopolysaccharides, bind to Toll-like-receptor-4, stimulate IL-18 production, and started the production line of IL and TNF. This leads to downstream effects including relaxation of the lower esophageal sphincter as well as decreased gastric motility.
It has been hypothesized that bacterial biofilms may be involved in the pathogenesis of reflux esophagitis. A biofilm is an organized microbial community that produces defense factors and adhesion molecules that enhance the survival of the local microbial community. Biofilms have been implicated in the pathogenesis of various disease states in the gastrointestinal tract, especially in the oral cavity and colon, while the extent to which it contributes to esophageal disease remains unclear.
Microbiological variation may help explain the phenotype of reflux esophagitis:
Variation in local microbiota composition may help explain the mechanistic and clinical differences between the two patterns pattern of reflux esophagitis. A recent study showed a distinct microbiome in NERD patients when compared with controls and RE subjects. The NERD microbiome composition shifted to Proteobacteria (Neisseria oralis and Moraxella spp) and Bacteroidetes (Bacteroides Uniformis, Capnocytophaga spp., and Prevotella pallens), and away from Fusobacteria (Leptotrichia) and Actinobacteria (Rothia). Several genera Firmicutes were reduced in NERD (Peptococcus and Moryella). However, the increasing abundance of Dorea spp. Leads to a higher overall Firmicutes component compared to the control. The same study speculated that an increase in sulfate-reducing Proteobacteria spp. and Bacteroidetes spp. together with hydrogen produces Dorea spp. implicated in a mechanistic role in visceral hypersensitivity in NERD. In addition, patients with RE had a decrease in Mogibacterium spp. , Streptococcus infantis , Solobacterium moorei) and increased Gram-negative bacteria (Leptotrichia spp.) and Proteobacteria (Marivita, Neisseria, and Mesorhizobium spp.).
Changes in composition in the esophageal system can also occur as a response to environmental factors. High-fat diets are strongly associated with changes in local mucositis in canine models. It is hypothesized that the changes in the light microenvironment seen with a high-fat diet lead to increased intestinal epithelial interactions and promote the progression of the inflammatory response. In addition, these dietary changes have been shown to contribute to an increased Firmicutes/Bacteroidetes ratio in the colon, which is associated with obesity and an inflammatory response characterized by the expression of the chemokine IL-8 . Other dietary factors, including consumption of insoluble carbohydrates, may contribute to reflux through influencing gastric and esophageal tone. Bacterial metabolism of carbohydrates into short-chain fatty acids (SCFAs) has been associated with an increase in peptide YY and oxyntomodulin, inhibition of gastric motility and lower esophageal sphincter function.
Barrett's esophagus (Barrett's esophagus)
Barrett's esophagus is a disease state characterized by stratified squamous metaplasia of the distal esophageal epithelium, and is often associated with longstanding reflux esophagitis. The incidence of Barrett's esophagus has increased since the mid-20th century, which is thought to be related to acute population changes in esophageal microbiota composition following the introduction of antibiotics [96]. Activation of the lipopolysaccharide-toll-like-receptor 4-NF-κB pathway through dysbiological alterations may contribute to inflammation and malignant transformation.
Mechanism of inflammatory pathogenesis of Barrett's esophagus:
The pattern of reflux-related inflammation seen in reflux esophagitis is also present in Barrett's esophagus. However, there are a number of specific dysbiological changes that also contribute to inflammation-mediated metaplasia. Barrett's murine model of the esophagus demonstrated that focal cytokine expression, especially in the distal esophagus, can lead to an inflammatory response in gastric stem cells, ultimately promoting the formation of columnar epithelium. . In addition, a similar anti-inflammatory response involving lipopolysaccharide and Toll-like-receptor -4 binding may be more noticeable in the distal esophagus with the increased presence of gram-negative bacteria, and especially contribute to the transformation of production.
The proinflammatory cytokine IL-1b, has been shown to be highly expressed in Barrett's esophagus:
The precursor of IL-1b requires proteolysis by caspase-1. Caspase-1 also enables the apoptosis of cells which may induce further inflammation. The functional role of caspase-1 is activated by inflammatory subtypes containing pattern recognition receptors (PRRs). PRR captures specific pathogen-associated molecular patterns (PAMPs) from bacteria and damage-associated molecular patterns (DAMPs) from injured cells. Once the PRR recognizes a given PAMP or DAMP, a large inflammatory complex activates caspase-1, initiating the inflammatory response pathway. One study demonstrated that lipopolysaccharides in Barrett's epithelial cells have been shown to prime and activate NOD-like 3-receptor protein pro-inflammatory responses and induce a cascade of proinflammatory responses. apoptosis. These data suggest that Barrett's esophagus survives through a chronic inflammatory response to focal cytokine release, initiated at the molecular level of the microbiome and associated mucosal cell responses. mandarin. Role of the microbiome in Barrett's esophagus:
Patients with Barrett's esophagus were found to have both similarities and differences in the esophageal microbiome profile when compared with those with inflammation reflux esophagitis. Using the original clustering model, the microbial community transition from Type I to Type II likely contributed to local inflammation. More recently, it has been shown that specific flora plays an important role in its pathogenesis. Fusobacteria, Neisseria spp. , and Campylobacter spp. was associated with Barrett's esophagus when compared with controls.
In addition, comparison of hyperplastic tissue with adjacent normal regions demonstrates reduced α diversity and reduced prevalence of Bacteroidetes including Prevotella spp. Distinct compositional differences in specific phyla occur along the NERD pathway, Barrett's esophagus, and esophageal adenocarcinoma. While Proteobacteria were overrepresented in NERD (Bacteroidetes to a lesser extent), the esophageal microbiota of Barrett and RE demonstrated an increase in Fusobacteria and Proteobacteria. The transition to esophageal adenocarcinoma microbiota represented an increase in Firmicutes, a decrease in NERD and Barrett's esophagus. This increase may reflect the increase in Firmicutes/Bacteroidetes ratio seen in obesity and rectal adenocarcinoma, as fermentation is impaired by Bacteroidetes spp. dietary fiber to anti-inflammatory SCFAs that may contribute to local inflammatory efflux.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.