This is an automatically translated article.
The article is made by Dr. Nguyen Khanh Hoa - Research project manager - Vinmec Institute of Stem Cell and Gene Technology.
Gut microbiota plays an important role in the absorption of nutrients and maintenance of metabolism, potentially influencing the development of metabolic disorders in humans such as obesity and type 2 diabetes. In this column for the journal Cell Metabolism, Krisko et al. (2020) demonstrated that the gut microbiota regulates glucose homeostasis through hepatic glucose biosynthesis and not through thermogenesis adipose tissue as previously reported.
1. Intestinal microbiota and its relationship to glucose homeostasis and energy metabolism
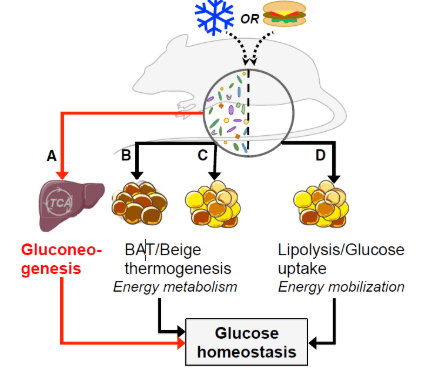
Hệ vi khuẩn ruột và mối liên quan của nó với Cân bằng Glucose nội môi và chuyển hóa năng lượng theo quan niệm cũ (mũi tên màu đen) và mới (mũi tên màu đỏ)
(A) Microbial metabolites control hepatic glucose generation, demonstrated by Krisko et al. (2020) in Cell Metabolism. (B and C) (B) gut microbiota actively supports thermogenic brown adipose tissue (BAT) and (C) additional beige adipose tissue invasion, both contributing to energy metabolism. (D) Beige adipose tissue was also seen in bacteria-deficient mice, possibly related to endogenous energy mobilization, as seen under fasting conditions. All mechanisms are likely to influence the rat glucose homeostasis system.
Metabolic disorders have become one of our society's most challenging burdens, affecting quality of life and profoundly impacting the health care system. Therefore, it is of the utmost importance that the scientific community continue to invest great efforts in the prevention and treatment of metabolic disease. Basic understanding of metabolic mechanisms often begins with laboratory studies in mice before this knowledge is translated to humans. Complex cellular interactions and molecular mechanisms enable organisms to maintain metabolic homeostasis influenced by genetics, age, and environmental factors. We do not seem to understand the roles of different organs in the pathogenesis and progression of metabolic disease, although it has been shown that the gut microbiota significantly influences these processes. The gut microbiome comprises a diverse microbial community in our digestive system that interacts with host metabolism and thereby increases the complexity of metabolism. In healthy individuals, the gut microbiota is in balance with the metabolic needs of the host. Environmental stressors, such as changes in nutrition and ambient temperature, have a significant impact on interactions between microbial and host metabolism, affecting body weight. body mass, thermoregulation, and glucose homeostasis. In contrast, metabolic disorders such as obesity, as well as host genetics, have a great deal of influence on the composition of the gut microbiota.
2. The interaction between gut microbiota and host metabolism
Hệ vi khuẩn đường ruột
With such complexity, it is not surprising that scientists' observations are often diverse, often conflicting about the outcomes of interactions between the gut microbiome and animal metabolism. owner . In the laboratory mouse experiment, this difference could be due to differences in the genetics of the mouse strain, sanitary status, differences in food and water supply and housing temperature: all contribute factors that influence the biology of the host and gut microbial communities. It is likely that the researchers could not control for all confounding factors, despite the desire of the scientific community to perform standardized experiments and thus provide only partial conclusions. Although this limitation can make it difficult to compare results between different laboratories, scientists also consider these cases positively; it is an opportunity to explore the full diversity of metabolic interactions and effects. In this respect, we should not forget that humans, unlike laboratory mice, have significant genetic differences, not living according to standards, in a sterile environment, but environmental conditions, lifestyle , their exposure is constantly changing. But, how does the gut microbiota communicate with distant organs? How does this association lead to no obesity and no type 2 diabetes? To this day, the details of this contact are puzzling. Important methods for investigating metabolic effects are to deplete the gut microbiota using a mixture of antibiotics, or to maintain mice in a germ-free environment, or to culture the microbiota between mice. .
Using these methods, the researchers hypothesized that the gut microbiota influences thermogenesis (brown fat) and energy metabolism, thereby indirectly controlling glucose homeostasis through enhancing burn glucose. These hypotheses are still conflicting, as some studies imply a positive effect of the gut microbiota on brown and beige adipose tissue to increase thermogenesis and energy use in cold while those in others saw the appearance of beige adipose tissue when mice were deficient in gut bacteria. All of these studies to date have suggested that the effect of gut bacteria is on thermogenic adipose tissue to balance glucose homeostasis.
hệ vi khuẩn ruột có thể tác động vào mỡ nâu sinh nhiệt
In this study, Krisko et al (2020) determined the relationship between gut microbiota, thermogenesis and glucose homeostasis (Figure 1) by excluding the need for increased energy metabolism and adipose tissue are thermogenic and instead demonstrate that the gut microbiota regulates glucose homeostasis through hepatic glucose biosynthesis. As a significant result, the authors did not ignore colonic dilation and intestinal elongation in two mouse models of microbial depletion, antibiotic-mediated and non-reproductive mice. In their detailed analysis, they appreciated that, for the first time, an extra volume of water in the stool confounds the results of energy use, and therefore should only be based on the total metabolic weight of the organ. work. Eliminating mass differences, either from calculation or from experimentation by ablation, eliminating differences in energy use, focusing on the question of whether gut microbiota affects energy use .
Combined with the energy use thermogenesis data, Krisko and colleagues observed no change in adipose tissue. The difference with previously observed in adipose tissue remains puzzling. But is it possible to decipher the data for a unified explanation? Declining gut microbiota likely sent the mice to different states of energy deprivation, depending on their condition. If the host micro-organisms are not cold enough, mice without the microorganism may develop heat-inhibited adipose tissue, and when transplanted cold-adapted bacteria may support additional heat capacity through beige adipose tissue. However, beige adipose tissue was also seen in response to microbial depletion. Here, brown fat may be primarily associated with an endogenous mechanism that enhances fat mobilization rather than energy expenditure (Figure 1). In another experiment in mice, the effects of microbial depletion may not even require the participation of beige adipose tissue. Differences in results from one laboratory to another can to some extent be attributed to differences in rat baseline standards and rat strains. Importantly, the observed reduction in blood glucose levels of gut microbiota-depleted rats was reported by Krisko et al. (2020) is consistent with previous findings by others regarding changes in insulin sensitivity and thermogenic activity.
Therefore, it can be concluded that glycemic control can function independently of the energy consumption of thermogenesis adipose tissue, shifting the focus to other organs. The authors of this report recently found that the microbiota regulates glucose biosynthesis in the liver to maintain blood glucose levels. Furthermore, Krisko and colleagues using identified metabolites that enhanced biosynthesis by performing comprehensive metabolomics analysis provided an important finding on the origin of the bioactive microorganisms. molecular science. Additional studies will be needed to establish causality, which may involve selective microbial transfer or genetic manipulation of the transporter liver. Overall, the publication of Krisko et al (2020) offers new paradigms with the importance of the field of metabolism: first, the assessment of intestinal inert mass to determine the exact The determination of metabolic rate, and second, the gut microbiota-hepatic axis in the control of glucose biosynthesis, prompt further discoveries of the molecular mechanisms that reside in the liver to elucidate the metabolic pathways. observations into clinical intervention strategies.
Customers who need examination and treatment can directly go to Vinmec International General Hospital nationwide or for more information, please contact the hotline HERE.