This is an automatically translated article.
Posted by Doctor Mai Vien Phuong - Department of Medical Examination & Internal Medicine, Vinmec Central Park International General Hospital.
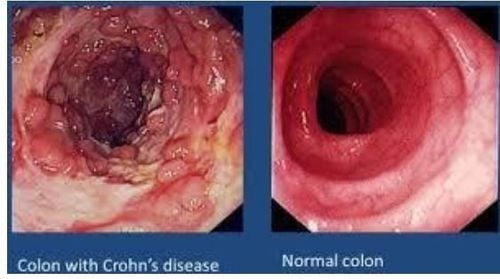
Patients with ulcerative colitis often have abdominal cramps, diarrhea, weight loss, and bleeding from the intestinal tract. The two types of ulcerative colitis are Crohn's disease and ulcerative colitis. The following article deals with a credible study that evaluated the role of bifidobacteria in UC remission.
1. The role of gut microbiota in ulcerative colitis
The pathogenesis of UC remains unknown. Genetic and environmental factors clearly contribute. Luminal bacteria may play an important role in the onset and persistence of chronic UC. Thousands of endogenous bacteria live in the large intestine and may be essential in a number of medical disorders.
UC animal models show that colitis does not occur in a germ-free environment. In UC in humans, inflammation occurs in the parts of the intestine where the bacterial concentration is highest. Furthermore, the terminal ileum, cecum and rectum are relatively static, allowing the mucosa to be in longer contact with the contents. The enhanced permeability of the mucosa may play an important role in the maintenance of chronic inflammation due to genetic predisposition or direct exposure to bacteria or their products. To date, no specific microorganism has been directly implicated in the pathogenesis of UC. However, gut flora analysis revealed differences in the composition of this flora compared with healthy controls. In UC, Bacteroides, Eubacteria, Peptostreptococci and anaerobic bacteria increased, while the number of Bifidobacteria decreased significantly. Over the past few years, the use of probiotics in IBD and other intestinal disorders has gained attention. Controlling colonic bacteria with antibiotics and probiotics has been shown to be more effective and tolerable than immunosuppressants.
Probiotics and prebiotics have a role in the prevention or treatment of certain diseases. The mechanisms by which dysregulation may play a central role in the initiation and persistence of inflammatory bowel disease have been discussed. BIFICO probiotic capsules are used for Enterococci, Bifidobacteria, Lactobacilli three viable bacteria, probiotics such as VSL
3 can also be used to maintain clinical remission and prevent relapse in those patients with recurrent or chronic pouchitis. Treatment with anti-inflammatory drugs and immunomodulators is commonly required for patients with antibiotic-resistant chronic pouchitis Probiotic Lactobacillus rhamnosus GG (LGG) has been shown to be beneficial for the treatment of associated diarrhea. to viruses and antibiotics. Nitric oxide (NO) is involved in protective mechanisms in the gastrointestinal tract and may contribute to some of the beneficial effects of probiotics. LGG induces NO in J774 macrophages and in human T84 colon epithelial cells through iNOS induction by a mechanism involving the activation of the transcription factor NF-κB. Low-level iNOS induction and NO synthesis may participate in the protective activities of LGG in the gastrointestinal tract.

Recent results support the concept that gut bacteria can induce endogenous signals with pathogenic roles in hepatic insulin resistance and NAFLD, and suggest novel therapies for these this common condition. Many authors found that high concentrations of Gram-positive bacteria in the luminal flora can effectively prolong the recurrence time of ulcerative colitis.
2. Role of probiotics on NF-κB . transcription
It is well known that the transcription factor NF-κB plays an important role in the expression of LPS-induced inflammatory factors. Because NF-κB activation can lead to enhanced expression of proinflammatory cytokines, chemokines, inflammatory enzymes such as inducible NO synthase (iNOS) and cyclooxygenase (COX-2), adhesion molecules and inflammatory receptors that modulate NF-κB activation may provide a direct way to inhibit inflammatory mediators.
NF-κB activation is activated by phosphorylation of an inhibitory subunit, IκB. In unstimulated cells, NF-κB is sequestered in the cytoplasm through interaction with the inhibitory proteins IκB a and IκBa. In response to proinflammatory stimuli, IκB is first phosphorylated at its N-terminal region by a large multi-kinase complex, then polyubiquitinylayed, and finally degraded by the proteasome. The released NF-κB complex can translocate to the nucleus where it initiates gene transcription upon binding to its cognate DNA motifs in regulatory segments of the TNF-α gene and other target genes involved. related to inflammation and immunity.
At the same time, the authors also discovered the effect of probiotics on the initiation and prolongation of the inflammatory process. The expressions of IκB in the cytoplasm and NF-κB in the nucleus were detected by western blot. The results showed that the probiotic effectively inhibited the breakdown of IκB protein. Less NF-κB translocation into the nucleus could be detected by western blot in the treatment group. NF-κB activation was analyzed by EMSA, which also showed that more NF-κB proteins were significantly activated in the control group compared with the probiotics group.
3. Role of mucosal immune response
Recent data have demonstrated that mucosal immune responses are relevant in IBD patients. The nuclear transcription factor NF-B is a key regulator of the inducible expressions of many genes involved in immune and inflammatory responses in the gut. Stimuli such as oxidative stress, cytokines (IL-1, IL-6, TNF-alpha), bacteria and viruses can release NF-κB from their dormant cellular form into the nucleus.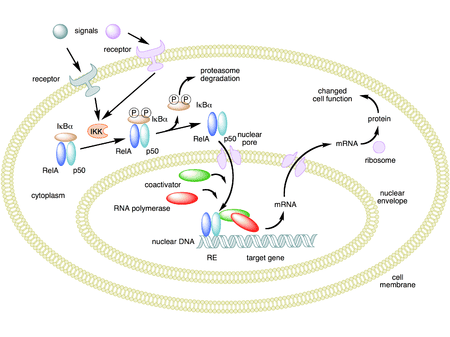
Inhibitory cytokines can block the activation of NF-κB. More potent and selective therapeutic strategies with anti-p65 inhibitor, proteasome and viral alpha I kappa B expression vector have been aimed at blocking NF-κB activation in mucosal macrophages and cells T lymphocytes. However, NF-κB regulatory genes are also involved in epithelial cell survival responses. In the study of several authors, the expression of proinflammatory cytokines such as TNF-α, IL-1β affected by the activation of NF-κB was clearly inhibited by probiotics and the mRNA expression of cytokines Anti-inflammatory IL-10 is increased by the action of probiotics.
Conclusion
Probiotics supplementation is useful in maintaining remission and preventing UC recurrence.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.