This is an automatically translated article.
Menopause is one of the stages that causes many unpleasant symptoms, due to hormonal deficiencies in women. One of the strides to improve the quality of life of menopausal women is the introduction of hormone replacement therapy. However, studies of its use have shown negative health effects, particularly an increased risk of breast cancer in women. So does hormone replacement therapy (HRT) really increase breast cancer risk?
1. What is hormone replacement therapy?
Hormone replacement therapy (HRT), also known as menopausal hormone therapy (Menopause Hormone Therapy) or postmenopausal hormone therapy (Postmenopausal hormone therapy). This is a therapy that uses external hormones to treat symptoms that occur during menopause in women. These symptoms include atrophic vaginitis and vaginal dryness, osteoporosis, and other psychological symptoms. These symptoms are caused by a drop in sex hormone levels during menopause.
The main hormonal drugs used in HRT to treat menopausal symptoms are estrogen and progestogen. Progestogen is often used in combination with estrogen in women with an intact uterus (without uterine pathology or hysterectomy for many reasons). For estrogen alone (without progesterone) therapy is associated with endometrial hyperplasia, endometrial cancer, and progestogens help prevent these risks. Androgens, like testosterone, are also sometimes used in HRT.

Hormone replacement therapy mainly focuses on replacing the estrogen your body no longer makes after menopause. There are two main types of estrogen therapy:
Systemic hormone therapy. Systemic estrogen — which comes in pill, skin patch, ring, gel, cream, or spray form — typically contains a higher dose of estrogen that is absorbed throughout the body. It can be used to treat common symptoms of menopause. Low dose vaginal product. Low-dose estrogen vaginal preparations — which come in creams, tablets, or rings — minimize the amount of estrogen absorbed by the body. Therefore, low-dose vaginal preparations are generally used only to treat vaginal and urinary symptoms of menopause. If you haven't had a hysterectomy, your doctor will usually prescribe estrogen along with progesterone or a progestin (progesterone-like drug). This is because estrogen-only HRT therapy, when not balanced by progesterone, can stimulate the growth of the uterine lining, increasing the risk of endometrial cancer. If you have had a hysterectomy (removal of the uterus), you may not need to take a progestin.
Trắc nghiệm: Những lầm tưởng và sự thật về ung thư vú
Ung thư vú có tỷ lệ tử vong cao nhất ở nữ giới khiến họ rất lo sợ bản thân mắc phải căn bệnh này. Tuy nhiên, không ít chị em có những hiểu biết thái quá về ung thư vú. Thử sức cùng bài trắc nghiệm sau sẽ giúp bạn loại bỏ được những nghi ngờ không đúng về căn bệnh này.
Bài dịch từ: webmd.com
2. Hormone replacement therapy and breast cancer risk.
Hormone replacement therapy (HRT) is taken to relieve menopausal symptoms such as night sweats and hot flashes. But it has long been recognized that HRT is associated with an increased risk of breast, endometrial and ovarian cancer, as well as an increased risk of cardiovascular problems such as an increased risk of blood clots.

These women still had an increased risk of breast cancer more than 10 years after they stopped taking HRT. The risk is higher with HRT that combines estrogen and progestogen than with estrogen-only HRT.
For the general population, an average of 63 women out of every 1,000 women aged 50 to 69 years will develop breast cancer. The incidence and development of breast cancer between the ages of 50 and 69 is:
63 per 1,000 women who have never used HRT 83 per 1,000 women who have used combined HRT (estrogen and progesterone) for 5 years from 50 age 68 per 1,000 women using estrogen-only HRT for 5 years from age 50 Using combined HRT for 5 years causes an additional 15 to 20 cases of breast cancer per 1,000. Estrogen-only HRT increases the risk of endometrial cancer, so is usually only offered to women who have had a hysterectomy (removal of the uterus).
Tibolone (Livial) is another type of HRT that contains steroids that act like estrogen and progesterone. Tibolone users also have an increased risk of breast cancer, but probably less than combined HRT users.
The Medicines and Healthcare products Regulatory Agency (MHRA), the UK health product safety regulator, has advised women concerned about the risk of breast cancer to use it. HRT should be consulted and consulted with a specialist before using
3. Why does hormone replacement therapy increase breast cancer risk?
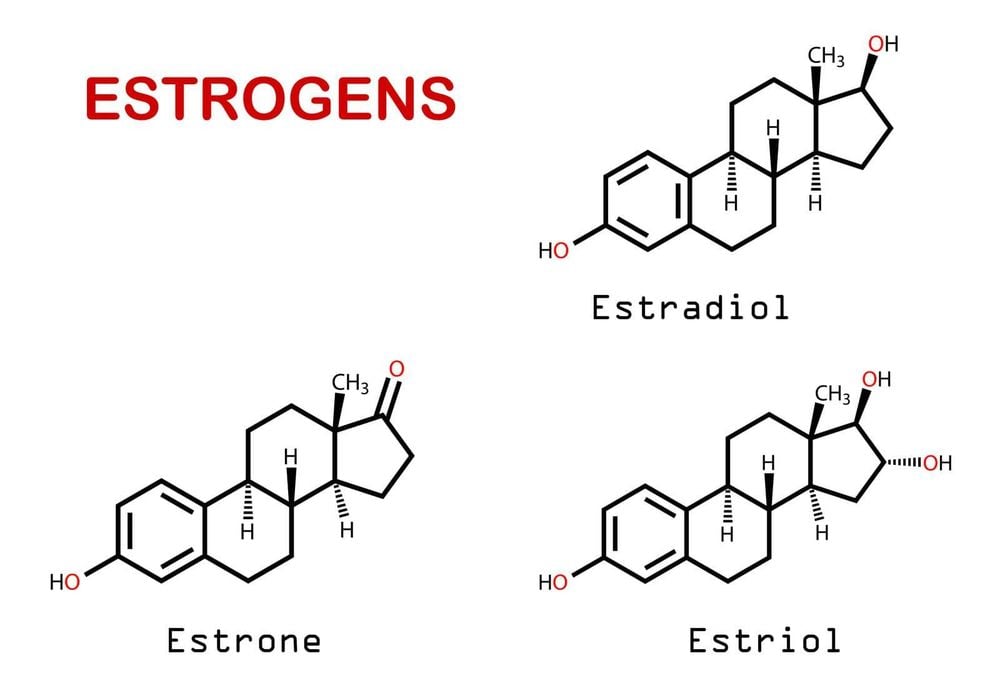
3.1. Estrogen and breast cancer The exact mechanism for estrogen's association with breast cancer has not been fully elucidated. Theories are that when estrogen binds to estrogen receptors, it stimulates cell proliferation. As cells proliferate, there is a risk of errors in the cyclical DNA replication. If these errors are not corrected, these mutations will lead to the transformation that leads to breast cancer. Another theory is that estrogen promotes already existing tumors rather than causes cancer. This is supported by observational studies in which cases in hormone users were noted after 1 year and the risk returned to baseline after discontinuation of HRT. A third hypothesis is that metabolites of oral estrogen react with the DNA of breast tissue to cause cancer. It is very likely that more than one of these mechanisms is involved in breast cancer development. To date, there have not been strong enough data to prove that estrogen is a cause of breast cancer.
3.2. Progestin and breast cancer Many questions have been raised regarding the role of progesterone in breast cancer development. When using combined estrogen/progestin therapy, the risk of breast cancer is consistently higher than with estrogen alone. Many studies have evaluated the type of progestin used (natural versus synthetic), the method of use (cyclic or continuous), and whether the progestin is derived from testosterone. Some authors have concluded that only continuous use (eg, conjugated equine estrogens) is associated with an increased risk of breast cancer. A meta-analysis of observational studies suggested that the relative risk of continuous progestin use was generally two to three times higher than that of cyclic progestin regimens.
Different derivatives of progesterone also seem to pose different risks. Several authors have shown that the non-progesterone properties of synthetic progestins increase breast tissue proliferation, decrease insulin sensitivity, and increase insulin-like growth factor activity. A French study found that natural progestins did not significantly increase the risk of breast cancer when compared with synthetic progestins for >2 years of use. Beneficial effects of natural progestins on the endometrium, brain, macrophages and arterial walls have been reported. Testosterone-derived progestins (eg, 19-nortesterone) have greater androgenic and estrogenic activities and, therefore, may increase breast cancer risk. Levononorgestrel is more androgenic than norethisterone, more androgenic than medroxyprogesterone acetate (MPA).
The exact mechanism of how progesterone increases breast cancer is still unknown. Maximum mitotic activity in breast tissue occurs during the mid to late luteal phase of the menstrual cycle, at the time when progesterone levels are maximal.
4. Recommendations regarding the use of HRT
After evaluating all available evidence, the PRAC (Pharmacovigilance Risk Assessment Committee) recommends the following for information on hormone replacement therapy use:
For combined estrogen and progestogen HRT, product information should be clearly reflect a higher risk of breast cancer after about 3 years of product use. The risk of breast cancer decreases over time after stopping HRT, but the length of time it takes to return to baseline depends on how long prior HRT was used. New studies have shown that this risk may persist for 10 years or more in women who have used HRT for more than 5 years. For low-dose vaginal estrogens, current evidence does not suggest an increased risk of breast cancer in women with no history of the disease. It is not yet clear whether this HRT regimen can be used safely in women who have had breast cancer in the past. The PRAC emphasizes that women should only use HRT to treat menopausal symptoms at the lowest effective dose and for the shortest possible duration, and that the information should be clearly stated on HRT products. In addition, women should also have regular checkups, including breast exams, according to current recommendations, and seek medical attention if any breast changes are noticed. If you have a need for consultation and examination at Vinmec Hospitals under the national health system, please book an appointment on the website for service.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
References: ncbi.nlm.nih.gov, breastcancer.org
See also:
Hormone replacement therapy: benefits and risks
Hormone replacement therapy and (HRT) menopause
Is hormone replacement therapy right for you?