This is an automatically translated article.
Cerepax 750 is an anticonvulsant indicated in the treatment of epilepsy. However, Cerepax 750 has some serious unwanted effects on the skin, blood, and mental health. So when using Cerepax 750, what should be noted to be effective and safe? Let's learn about Cerepax 750 in the following article.
1. What is Cerepax 750?
Cerepax 750 is a product of OPV pharmaceutical joint stock company, prepared in the form of orange film-coated tablets with the active ingredient Levetiracetam, content 750 mg.
Levetiracetam is an anticonvulsant chemically unrelated to other antiepileptic drugs. The mechanism of action of Levetiracetam is still unknown. Levetiracetam does not bind to peripheral tissues but is only locally bound to synaptic membranes in the central nervous system. Levetiracetam has no effect on normal nerve excitability but has an inhibitory effect on flare. The drug thus selectively prevents excessive synchrony of epileptic flare and seizure propagation.
2. Indications of the drug Cerepax 750
Cerepax 750 is indicated in the following cases:
Treatment of partial seizures in children 4 years of age and older and adults with epilepsy. Treatment of myoclonic seizures in children 12 years of age and older and adults with myoclonic epilepsy. Treatment of generalized seizures in children 6 years of age and older and adults with idiopathic generalized epilepsy.
3. Contraindications of Cerepax 750
Do not use Cerepax 750 in cases of hypersensitivity to levetiracetam or any of its ingredients.
4. Dosage and usage of Cerepax 750
How to use: Cerepax 750 can be taken with or without food.
Dosage:
- Treatment of partial seizures:
Children over 16 years old and adults: Initial dose is 1000mg/day (500mg/time x 2 times/day), maintenance dose can be increased by 1000mg /day every 2 weeks, maximum dose 3000mg/day.
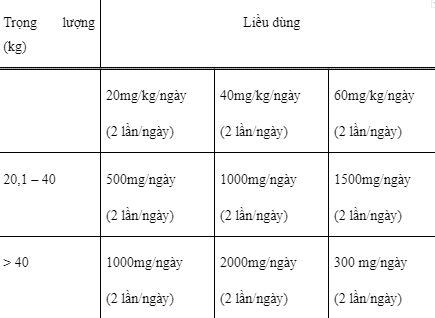
Children from 4 to 16 years old: Initial dose is 10mg/kg x 2 times/day, maintenance dose can be increased by 20mg/kg every 2 weeks, maximum dose is 60mg/kg/day.
Dosage for children is guided in the following table:
- Treatment of myoclonic seizures in children over 12 years of age and adults with myoclonic epilepsy: Initial dose is 1000mg/day (divided into 2 times/day) then dose may be increased by 1000mg/day every 2 weeks up to the recommended dose of 3000 mg/day.
- Treatment of primary generalized convulsions:
Children over 16 years of age and adults: Initial dose is 1000mg/day (divided into 2 times/day), then dose can be increased by 1000mg/day every 2 Maximum weekly dose is 3000mg/day.
Children from 6 years to 16 years: Initial dose is 2mg/kg/day (divided into 2 times/day), daily dose may be increased by 20mg/kg every 2 weeks up to the recommended dose of 60mg/kg /day (divided into 2 times / day).
The dose of Cerepax 750 used in patients with renal impairment is adjusted as follows:
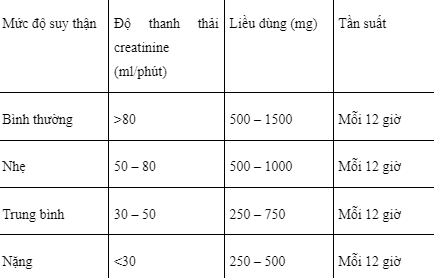
Do not stop suddenly when using Cerepax 750, must gradually reduce the dose of 1000mg / day every 2 weeks.
5. Undesirable effects of the drug Cerepax 750
When using Cerepax 750, patients may experience the following undesirable effects:
Common undesirable effects (frequency of ADR > 1/100):
Digestive system: vomiting, loss of appetite; Immune system: Infections; Musculoskeletal system: Neck pain; Nervous system: Asthenia, headache, dizziness, insomnia ; Psychiatric: depression, feelings of stress, abnormal behavior, antagonistic behavior, mood swings, mood disturbances; Respiratory system: Pharyngitis, cough, rhinitis; Other: pain, fatigue. Serious undesirable effects may occur:
Skin: Drug-induced toxic epidermal necrolysis, Stevens-Johnson syndrome; Blood: Thrombocytopenia, cell lines; Liver: Liver failure; Psychiatric: Suicidal ideation, suicide.
6. Notes when using Cerepax 750
Caution in patients with renal failure, dialysis, liver failure. During the use of Cerepax 750, if the patient has neurotic symptoms (hallucinations...), behavioral symptoms (anxiety, agitation, etc.), the dose should be reduced. Stopping Cerepax 750 suddenly may lead to an increase in seizure frequency. The safety of Cerepax 750 in children under 4 years of age has not been established. Levetiracetam crosses the placenta, so Cerepax 750 should not be used during pregnancy. Since Levetiracetam is excreted in human milk, consideration should be given to discontinuation or discontinuation of breast-feeding. Cerepax 750 may cause dizziness and drowsiness, so do not drive or operate machinery while taking this medicine. In the event of serious skin reactions (toxic epidermal necrolysis, Stevens-Johnson syndrome), the drug should be discontinued immediately and alternative therapeutic measures instituted. Patients receiving an overdose of Cerepax 750 may experience symptoms such as agitation, aggression, somnolence, decreased consciousness, respiratory depression, and coma. In this case, unabsorbed drug can be removed by induction of vomiting or gastric lavage, and dialysis may be considered in patients with renal failure. Keep the medicine out of the reach of children, do not use the medicine past the expiry date.
7. Drug interactions
To date, there is no clinical evidence of drug interaction between Cerepax 750 and other antiepileptic drugs such as Carbamazepine, Primidone, Gabapentin, Lamotrigin, Phenobarbital, Phenytoin and Valproate or with another drug.
The article has provided information about the uses, doses and precautions when using Cerepax 75. To ensure safety for your health and maximize the effectiveness of your treatment, you need to take Cerepax 75 exactly as directed by your doctor. Store Cerepax 750 in a cool, dry place, at a temperature below 30 0C, protected from light.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.