This is an automatically translated article.
The article is written by Master, Doctor Vu Hoang Huy - Emergency Doctor - Emergency Department - Vinmec Times City International General Hospital.Methanol, or wood alcohol, is a commonly used organic solvent. Methanol is a constituent of many synthetic organic substances and is the solvent of a large number of commercial products on the market, such as automotive window sprays, detergents, furnace fuels, and solvents. In many paints, varnishes, shading agents and solutions in copiers,... Methanol has some physical properties quite similar to ethanol, is a colorless, volatile liquid, with a slight odor and taste close to that of ethanol. But it is very toxic and cannot be used as a drink.
1. Overview of methanol poisoning
Mortality rate from methanol poisoning is still very high (from 41.2% to 57.1%) and survivors often have severe sequelae, especially eye sequelae. Severe methanol poisoning and death are associated with severe metabolic acidosis, central nervous system damage, optic neurotoxicity, respiratory failure, and circulatory failure. Organic acids, mainly formic acid, and free radicals generated from methanol metabolism cause dysfunction and cell death.Treatment of acute methanol poisoning needs to be conducted early, with the right method to save the patient's life and limit complications. The key issues in the treatment of acute methanol poisoning are dialysis and specific antidotes. Hemodialysis to remove methanol and metabolites and correct acidosis. The main antidotes used include ethanol and fomepizole, which prevent the conversion of methanol to formic acid.
2. An overview of ethanol
Ethanol has the chemical formula C2H5OH, is a liquid, colorless, rapidly soluble in water, very poorly bound to proteins, has a volume of distribution of 0.6 l/kg, molecular weight 46, specific gravity 0.7939 g/ml, excreted by dialysis.2.1. Absorption and Distribution of Ethanol Ethanol is rapidly absorbed from the gastrointestinal tract, mainly in the duodenum. The presence of food reduces and slows the absorption of ethanol. Ethanol is distributed in all aqueous tissues with a volume of distribution approximately 0.6 - 0.7 l/kg. Ethanol is rapidly distributed across the placenta and the blood-brain barrier.
2.2. Ethanol metabolism and elimination In the human body, the liver metabolizes 90-98% of ethanol, which is absorbed through three enzyme systems: Alcohol dehydrogenase (ADH) has a major role, the microsomal ethanol oxidation system (MEOS), catalase participates very little. Meanwhile, the kidneys and lungs excrete a part of the ethanol unmetabolized.
Zero-order kinetics, i.e., metabolism is not concentration dependent, except that ethanol concentrations are too low (< 10-20 mg/dl) or too high (> 200-300 mg/dl). The typical elimination rate of ethanol is about 15 - 20 mg/dl/h in healthy subjects, and ranges from 10 to 34 mg/dl/h. The rate of elimination of ethanol is generally higher in alcoholics than in non-alcoholics because in alcoholics the MEOS system (especially the cytochrome P450 enzyme) is more induced, resulting in increased activity.
3. The detoxification mechanism of ethanol in the treatment of acute methanol poisoning
In the body, ethanol is metabolized in the liver similarly to methanol by the enzyme ADH. However, ethanol has about 7-10 times greater affinity for the ADH enzyme than methanol. Therefore, if co-existing in the blood, ethanol will metabolize first, prolonging the half-life as well as prolonging the presence of methanol, pending dialysis and resuscitative measures to remove methanol.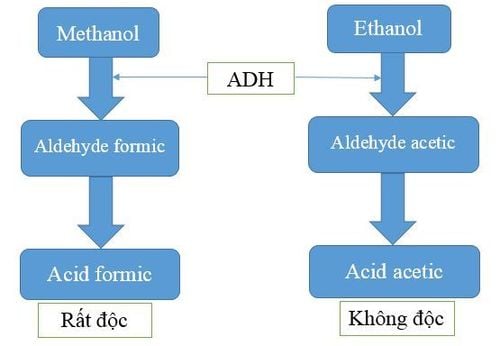
About how to calculate ethanol dose, there are many authors giving different dosages and calculation methods. However, the difference is not much.
In 1981, author Peterson CD recommended oral ethanol dose of 0.6 - 0.8g/kg, maintenance dose in dialysis is 250 - 350 mg/kg/hour and dose 110 - 130 mg /kg/hour without hemodialysis, the specific dose must be individualized based on the measured ethanol concentration. Based on the formula to calculate the estimated ethanol dose for blood ethanol from 100 - 200mg/dl, adjust the ethanol dose according to experience:
K0= (Vmax)(Css)/(Km+ Css). KD= K0 + (D)(Css). K0: Estimated ethanol concentration. KD: Estimated ethanol concentration in hemodialysis. Css: Desired ethanol concentration. Vmax: Ethanol elimination rate (124mg/kg/hour, average 75-175mg/kg/hour). Km: Michaelis-Menten coefficient (13.8 mg/dl). D: Ethanol dialysis. In 2002, the American poison control center issued a recommendation for ethanol dosage in acute methanol poisoning, this view also agrees with some other authors, specifically in the following table:
| Liều lượng | Dung dịch truyền TM 5% | Dung dịch truyền TM 10% | Dung dịch uống 20% |
| Liều ban đầu | 15(ml/kg) | 7,5(ml/kg) | 4(ml/kg) |
| Liều duy trì ở người không nghiện rượu | 2 – 4(ml/kg/giờ) | 1 – 2(ml/kg/giờ) | 0,5(ml/kg/giờ) |
| Liều duy trì ở người nghiện rượu | 4 – 8(ml/kg/giờ) | 2 – 4(ml/kg/giờ) | 1(ml/kg/giờ) |
| Liều duy trì khi lọc máu ở người không nghiện rượu | 4 – 7(ml/kg/giờ) | 2 – 3,5(ml/kg/giờ) | 1(ml/kg/giờ) |
| Liều duy trì lọc máu ở người nghiện rượu | 6 – 10(ml/kg/giờ) | 3 – 5(ml/kg/giờ) | 2(ml/kg/giờ) |
3.2. Some undesirable effects of ethanol Central nervous system effects: Ethanol causes central nervous system effects, ranging from mild drowsiness or euphoria to coma and respiratory depression.
Effects on the digestive tract: Increase transaminase enzyme: Expression of increased AST, ALT may be seen when treated with ethanol, especially in patients with viral hepatitis, cirrhosis.
Inflammation of the stomach lining: Manifested by bloating, epigastric pain, belching, heartburn. This problem causes decreased absorption of ethanol into the bloodstream.
Gastrointestinal bleeding: Gastrointestinal bleeding, duodenal but rare.
Acute pancreatitis : A serious but rare complication.
Some other undesirable effects: Ethanol can cause hypoglycemia, especially in children and malnourished patients.
Electrolyte disturbances: Possible hyponatremia and hypokalemia, but the mechanism is unclear.
Although the authors concluded that ethanol was a safe and effective antidote, it still had some potential for toxicity. Specifically in the table below:
| Nồng độ ethanol | Triệu chứng |
| <50mg/dl | Nói nhiều, cảm giác hạnh phúc. |
| 50-150mg/dl |
Lời nói, cảm xúc, vận động chậm chạp. Suy giảm thị lực nhẹ. |
| 150-300mg/dl | Nhìn mờ, mất nhận thức và cảm giác, không phối hợp, thời gian phản ứng chậm. |
| 300-500mg/dl | Nhìn mờ hoặc nhìn đôi, có thể hôn mê, hạ nhiệt độ, hạ đường máu, co giật. |
| ≥ 500 mg/dl | Hôn mê, suy hô hấp, giảm phản xạ, hạ huyết áp, hạ đường máu, tử vong do suy hô hấp, suy tuần hoàn, hoặc do sặc phổi. |
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.