This is an automatically translated article.
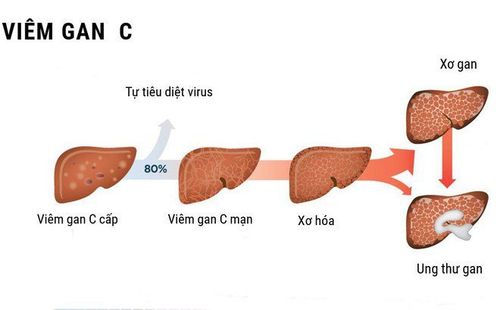
Zepatier has the main ingredients Elbasvir and Grazoprevir, used to treat chronic hepatitis C (HCV) genes type 1 and type 4. So what are the uses, doses and precautions when using Zepatier?
1. What effect does Zepatier have?
Zepatier has the main ingredients Elbasvir 50mg and Grazoprevir 100mg. Elbasvir is an inhibitor of HCV NS5A, required for viral replication and virion formation. Grazoprevir is an inhibitor of the HCV NS3/4A protease, an enzyme important for the proteolytic cleavage of HCV-encoded polyproteins and essential for viral replication. Zepatier is used to treat chronic hepatitis C (HCV) genotypes 1 and 4 in adults.
2. How to use, dose of Zepatier
How to use:
Zepatier is used orally. The patient can take it with or without food. Dosage:
The specific dose of Zepatier in the treatment of chronic hepatitis C is as follows:
Genotype 1a:
Untreated patients without cirrhosis: Take 1 tablet, once daily for 12 weeks; After kidney transplant, treatment-nave patients or non-direct antiretroviral therapy-treated patients without cirrhosis/compensated cirrhosis: Oral: 1 tablet, once daily for 12 weeks. Genotype 1b:
Untreated patients without cirrhosis or with compensated cirrhosis: Take 1 tablet, once daily for 12 weeks; After kidney transplant, treatment-nave patients or non-direct antiretroviral therapy-treated patients without cirrhosis/compensated cirrhosis: Oral: 1 tablet, once daily for 12 weeks. Genotype 4:
Untreated patients without cirrhosis or with compensated cirrhosis: Take 1 tablet, once daily for 12 weeks; After kidney transplant, treatment-nave patients or non-direct antiviral therapy-experienced patients without cirrhosis/compensated cirrhosis: Oral: 1 tablet, once daily for 12 weeks. Patients with hepatic impairment:
Mild hepatic impairment (Child-Pugh class A): No dosage adjustment required; Moderate or severe impairment (Child-Pugh class B or C) or decompensation: Contraindicated. Patients with renal impairment: No dosage adjustment of Zepatier
is required.
3. Contraindications of Zepatier
Contraindicated to use Zepatier in the following cases:
Moderate or severe liver failure (Child-Pugh class B or C); Patients with a history of decompensated liver disease; Concomitant use with OATP1B1/3 inhibitors; Hypersensitivity to Elbasvir, Grazoprevir or any component of the formulation.
4. What are the side effects of Zepatier?
Patients using Zepatier may experience the following side effects:
Frequency > 10%:
Nervous system: Fatigue, headache. Frequency 1 to 10%:
Liver: Increased serum alanine aminotransferase; Digestive: Diarrhea, constipation, dry mouth, stomach pain; Nervous system: Headache, anxiety, depression; Other: Itching, insomnia, muscle pain. Frequency <1%:
Hematology: Decreased hemoglobin ; Liver: Increased serum bilirubin, acute liver failure.
5. Notes when using Zepatier
Assessment of advanced cirrhosis and hepatocellular carcinoma should be performed prior to treatment. Assess potential drug interactions and patient compliance. Prior to initiating Zepatier, testing should be performed for evidence of current/previous HBV infection, quantification of hepatitis B surface antigen (HBsAg) and hepatitis B core antibody (anti-HBc). Elevated ALT is usually observed at 8 weeks. Women, Asian patients, and patients ≥ 65 years of age may be at higher risk of ALT elevation. If there are signs of fatigue, weakness, decreased appetite, nausea, vomiting, jaundice or discolored stools, patients should contact their doctor for advice. Liver function tests should be performed prior to treatment, at week 8 of therapy, and as clinically indicated. Consider discontinuing treatment if elevated ALT is associated with signs of hepatitis or increases in conjugated bilirubin, alkaline phosphatase, or INR. Rapid reduction of viral load during antiretroviral therapy may lead to improved glucose metabolism in diabetic patients, potentially leading to symptomatic hypoglycemia. Monitor blood glucose and inform patients of the risk of hypoglycaemia during treatment with Zepatier, especially during the first 3 months. Hepatitis B virus reactivation has been reported in some cases and has a case risk of fulminant hepatitis, liver failure, and death. Pregnancy: Use is not currently recommended for the purpose of reducing mother-to-child transmission of hepatitis C virus due to the lack of data on safety and efficacy. The decision to continue treatment in pregnant patients while taking the drug should be individualized after weighing the potential benefits and risks of therapy. Breast-feeding: Tell your doctor if you are breast-feeding or plan to breast-feed before taking this medicine.
6. Zepatier drug interactions
Drug interactions can change potency or increase the risk of side effects. Patients should inform their doctor or pharmacist of any medications they are taking, including prescription/nonprescription drugs and herbal products. Patients should not start, stop, or change the dose of any medication without first consulting a physician. The following are some drug interactions to watch out for when using Zepatier:
Inducers of CYP 344 such as Carbamazepine, Phenytoin, Phenobarbital may decrease the serum concentration of Elbasvir and Grazoprevir. CYP 344 inhibitors such as Erythromycin, Clarithromycin, Ketoconazole, Ritonavir may decrease serum concentrations of Elbasvir and Grazoprevir. Elbasvir and Grazoprevir may increase the serum concentration of Rosuvastatin. Rosuvastatin should only be initiated at a dose of 5 mg per day and a maximum dose of 10 mg per day of Rosuvastatin should be used during co-administration with Elbasvir/Grazoprevir. Monitor closely for evidence of toxicity of rosuvastatin eg, myopathy, rhabdomyolysis. Zepatier may increase serum concentrations of Elagolix, Estradiol and Norethindrone. Zepatier may reduce the effect of vitamin K antagonist anticoagulants. The article has provided general information about the drug Zepatier. However, the above information is for reference only, patients should contact their doctor or pharmacist for specific advice.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.