This is an automatically translated article.
Moritius 75mg is a drug manufactured by DAVI PHARM Co., Ltd., commonly used to treat central nervous system pain and peripheral neuropathic pain for adults, supporting the treatment of epilepsy, anxiety disorders. ..
1. What active ingredients does Moritius contain?
Ingredients in each Moritius hard capsule contains 75mg of Pregabalin and excipient system includes: lactose monohydrate, croscarmellose sodium, magnesium stearate,...
2. Uses of the drug Moritius
Effect of drug Moritius :
Moritius treatment of central and peripheral neuropathic pain in adults; Moritius adjunctive treatment of partial seizures, with or without secondary generalization; Moritius treats generalized anxiety disorder (GAD).

Moritius là thuốc dùng trong điều trị đau thần kinh, động kinh, lo âu
3. Dosage of the drug Moritius
Moritius treatment of neuropathic pain: Initial dose 150mg/day divided into 2-3 times, then depending on the specific response of the individual and tolerability, the doctor may increase the dose for the patient up to 300mg/day after about 3-7 days. The maximum dose of Moritius can be used up to 600mg/day after about 7 days if necessary; Moritius treatment of epilepsy: Initial dose 150mg/day divided into 2-3 times, then depending on individual response and tolerability, doctor may increase to 300mg/day after 1 week and maximum dose of 600mg/day after the next week; Treatment of generalized anxiety disorder: Initial dose of Moritius is 150mg/day, then depending on individual response and tolerability, doctor may increase to 300mg/day after 1 week, 450mg /day after the next 1 week and the maximum dose is 600mg / day. The pregabalin in Moritius did not alter the plasma concentrations of commonly used anticonvulsants and similarly did not affect the plasma concentrations of pregabalin. If pregabalin treatment is discontinued, it should be discontinued gradually over a minimum of 1 week.
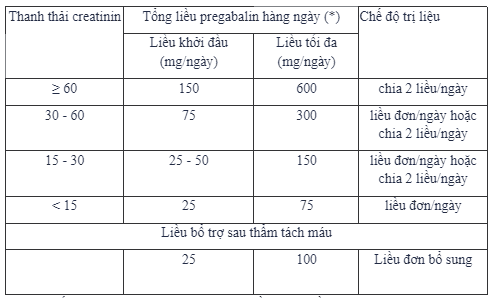
For patients with renal impairment: Pregabalin is excreted unchanged mainly by the kidneys, the clearance of pregabalin is proportional to the creatinine clearance, so the dose of Moritius should be reduced in patients with impaired renal function according to the renal function. creatinine clearance (ClCr). Pregabalin is effectively removed from the serum by hemodialysis (50% of the drug is removed after 4 hours) so for patients undergoing hemodialysis, the daily dose of Moritius should be adjusted according to function. renal function and in addition to the daily dose, the adjunctive dose of Moritius should be administered immediately after every 4 hours of hemodialysis.
For patients with hepatic impairment: No dose adjustment of Moritius is required in patients with hepatic impairment, Pregabalin is eliminated mainly in the urine unchanged, so patients with hepatic impairment do not have significant changes in drug concentrations in the liver. plasma; Children: The efficacy and safety of pregabalin in patients under 18 years of age have not been established; For the elderly (over 65 years old): No need to adjust the dose of Moritius for the elderly except in case of impaired kidney function; For pregnant women: There are no adequate data on the use of pregabalin in pregnant women, therefore Moritius should not be used during pregnancy unless the potential benefits outweigh the potential risks; For lactating women: It is not known whether pregabalin is excreted in human milk, however, this active ingredient is present in rat milk, so do not breastfeed while taking Moritius.
4. Contraindications of Moritius
Contraindicated in patients with hypersensitivity to any of the ingredients of Moritius.
5. Notes when using Moritius
Patients with rare hereditary problems of galactose intolerance, lactase deficiency or glucose - galactose malabsorption should not take Moritius ; Some diabetics who show signs of weight gain while taking pregabalin need to adjust the use of hypoglycemic agents; Treatment with pregabalin often causes dizziness and somnolence, the patient should be cautious until familiar with the possible effects of Moritius; Hypersensitivity reactions to pregabalin may cause angioedema, in which case Moritius should be discontinued immediately upon symptoms of swelling of the face, mouth or upper respiratory tract; Treatment with pregabalin can cause dizziness and somnolence, and an increased incidence of unexpected events (falls) in the elderly due to loss of consciousness, confusion, or mental decline, therefore patients should be cautious with this medication. the effect of the drug Moritius ; In controlled studies, pregabalin-treated patients had a higher incidence of blurred vision than placebo (placebo). Accordingly, adverse ocular effects including loss of vision, blurred vision or vision changes are usually transient and may be reduced or reversible upon discontinuation of Moritius; There have been reports of patients receiving pregabalin with congestive heart failure, which is common in the elderly with cardiovascular impairment, who received pregabalin for neurological disease, therefore caution should be exercised when pregabalin is administered to these patients. In this patient, this effect was reversible upon discontinuation of the drug; In the treatment of CNS pain due to spinal cord injury, the incidence of adverse events with Moritius is increased, especially on the CNS causing somnolence; There have been reports of suicide in patients treated with Moritius with antiepileptic drugs, the mechanism of action is unknown and the possibility of an increased risk cannot be excluded from the active ingredient pregabalin.
Sử dụng thuốc Moritius theo đúng chỉ định của bác sĩ
6. Moritius side effects
Side effects of Moritius are very common: dizziness, drowsiness; Common side effects of Moritius: appetite, euphoria, confusion, arousal, decreased libido, coordination disorder, tremor, dysarthria, memory loss, loss of concentration, paresthesia, blurred vision eyes, double vision, vomiting, dry mouth, constipation, flatulence, erectile dysfunction, abnormal gait, fatigue, peripheral edema, edema, weight gain...; Side effects of Moritius are uncommon: loss of appetite, hallucinations, panic attacks, restlessness, anxiety, depression, mood swings, severe insomnia, increased libido, lethargy, visual disturbances, eye swelling, decreased vision, eye pain, dry eyes, increased tearing, fast heartbeat, flushing, hot flashes, trouble breathing, rash, sweating; Rare side effects: neutropenia, decreased blood glucose; Side effects of Moritius but of unknown frequency: hypersensitivity, angioedema, allergic reactions. Discontinuation symptoms were observed in some patients when pregabalin was discontinued after long-term or short-term treatment with the following symptoms: insomnia, headache, nausea, diarrhea, flu, anxiety, depression, sweating and dizziness.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.