This is an automatically translated article.
Denatri main ingredient is Alfacalcidol. Alfacalcidol will be metabolized in the liver to 1,25-dihydroxy vitamin D3, which increases the absorption of calcium and phosphate in the intestines, promotes bone mineralization, reduces bone resorption, and regulates plasma parathyroid hormone levels. .
1. What is Denatri?
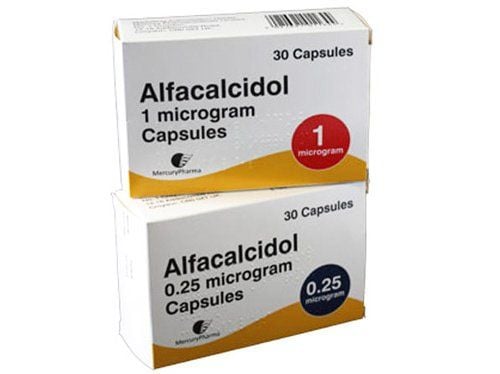
Denatri is formulated in the form of softgels with a content of 1mcg. The main mechanism of action of the drug is to increase the circulating concentration of 1,25-dihydroxy vitamin D3, thereby increasing the absorption of calcium and phosphate through the intestine, promoting bone mineralization, reducing bone resorption and bone pain, helping Regulates plasma parathyroid hormone levels. Denatri is used to treat osteodystrophy, parathyroid gland dysfunction (with bone disease), osteomalacia and rickets.
Alfacalcidol is fat soluble and generally well absorbed up to 100%. The presence of bile is necessary for the absorption of Alfacalcidol. In patients with reduced fat absorption, drug absorption may be reduced. After absorption, Alfacalcidol is rapidly hydroxylated in the liver to 1,25-dihydroxy vitamin D3, which acts as a regulator of calcium and phosphate metabolism. Thanks to this rapid metabolism, the therapeutic benefits of Alfacalcidol are almost identical to those of 1,25-dihydroxy vitamin D3. Alfacalcidol is transported in the blood bound to alpha-globulin. Alfacalcidol has a fairly rapid onset of action and a short half-life.
2. What are the effects of Denatri?
Denatri is indicated for treatment in the following cases:
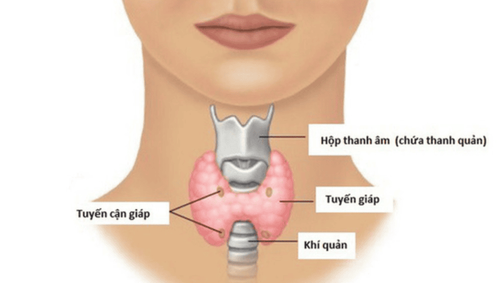
Renal osteodystrophy. Hyperparathyroidism with bone disease or hypoparathyroidism. Osteoporosis and rickets due to undernutrition or malabsorption. Osteoporosis and rickets are vitamin D dependent. Osteomalacia and vitamin D-resistant rickets with hypophosphataemia. Osteoporosis .
3. How to take Denatri
Denatri is prepared in the form of soft capsules and taken orally. The specific dosage of the drug is as follows:
Starting dose (except for patients with osteoporosis):
Adults: 1 μg/day. Children 12-18 years old: 1 μg/day and the dose can be adjusted if necessary. Children weighing 20 kg or more: Except for renal osteodystrophy: 1 μg/day. Renal osteodystrophy: 0.04 - 0.08 μg/kg/day. Children weighing less than 20kg: Denatri is not indicated because it is impossible to divide the dose accurately. Osteoporosis:
The usual dose of Denatri is 0.5 - 1 μg/day with or without calcium. Dosage is then adjusted according to the patient's response to avoid hypercalcemia. The dose of alfacalcidol can be increased to approximately 0.25 - 0.5 μg/day. Most adult patients respond to doses of 1-2 μg/day.
4. What to do in case of overdose of Denatri?
Overdose:
Symptoms of a Denatri overdose are fatigue, dizziness, dry mouth, nausea, vomiting, bone pain, muscle pain, joint pain, itching or palpitations. Treatment: Patients should discontinue Alfacalcidol treatment in the presence of hypercalcemia. Severe cases of hypercalcaemia may require supportive treatment and, if necessary, loop diuretics or corticosteroids. In case of acute poisoning, gastric lavage and/or mineral oil should be treated to reduce the absorption of Denatri and increase its excretion in the feces. Missed dose:
If a dose of Denatri is forgotten, the patient should take it as soon as he remembers. However, if it is almost time for the next dose, the patient should skip the missed dose and continue with the usual dosing schedule. Note: Do not take a double dose to make up for a missed dose of Denatri.
5. What are the side effects of Denatri?
When using Denatri, patients may experience some of the following undesirable effects:
Cardiovascular: increased blood pressure, arrhythmia. Central nervous system: Headache, hyperthermia, psychosis (rare), somnolence. Dermatology: Itching. Endocrine and metabolic: Decreased libido, hypercalcemia, hypercholesterolemia, hyperphosphataemia, weight loss. Digestive: Anorexia, constipation, digestive disorders, nausea, pancreatitis, vomiting. Genitourinary system: Nocturia. Liver: Elevated serum ALT and AST enzymes. Neuromuscular: Myalgia, bone pain, asthenia. Ophthalmology: Conjunctivitis, corneal calcification, photophobia. Kidney: Increased blood urea nitrogen, polyuria.
6. Notes when using Denatri
Denatri is contraindicated in patients with hypersensitivity to Alfacalcidol or any of its ingredients; patients with hypercalcemia, metastatic calcification, hypermagnesaemia or hyperphosphataemia. Excess vitamin D: Taking too much vitamin D can lead to over-suppression of parathyroid hormone (PTH), progressive or acute hypercalcemia, hypercalciuria, and hyperphosphataemia. Hypercalcemia: Monitor calcium levels closely. Patients with chronic renal failure are at increased risk of hypercalcaemia, therefore dose reduction may be required. Stop taking calcium supplements until calcium levels are normal. Denatri should be discontinued in case of hypercalcaemia in dialysis patients, and can be resumed with 50% of the previous dose 1 week after normalization of calcium levels. Chronic hypercalcemia can lead to general vascular and soft tissue calcification, exacerbation of nephrolithiasis, and is associated with increased mortality in adult patients with chronic kidney disease. In addition, prolonged hypercalcaemia can aggravate arteriosclerosis or valvular hardening, increasing the risk of arrhythmias. Use with caution in patients with calcification of lung tissue as it may lead to heart disease. Hyperphosphatemia: Monitor serum phosphate. In cases of progressive or persistent hyperphosphatemia, the use of phosphate-lowering agents may be necessary. Granulomatosis: Use with caution in patients with granulomatous disease (eg, sarcoidosis) due to increased sensitivity to vitamin D. Pregnancy: There are currently no data on the use of alfacalcidol in women. pregnant. Patients should be cautious when using during pregnancy because hypercalcaemia during pregnancy can cause congenital disorders in the baby. Lactation: 1,25-dihydroxyvitamin D concentrations may be increased in the milk of nursing mothers treated with alfacalcidol. This can affect the child's calcium metabolism. In general, the manufacturer recommends that Denatri should not be used in nursing women. Effects on ability to drive or use machines: Denatri has no or negligible influence on the ability to drive and use machines. the patient's machinery. Drug interactions: The following are some drug interactions that patients should be aware of when using Denatri: Denatri causes hypercalcemia and may cause cardiac arrhythmias in patients taking cardiac glycosides. Therefore, patients receiving concomitant cardiac glycosides and denatri should be closely monitored. Concomitant use of Denatri with Barbiturates or enzyme-inducing anticonvulsants may lead to a decrease in blood levels of Denatri. Therefore, it is necessary to increase the dose of alfacalcidol to achieve a therapeutic effect. Concomitant use with mineral oil, Colestipol, Cholestyramine, Sucralfate may reduce the absorption of Alfacalcidol. Patients taking calcium preparations or thiazide diuretics concomitantly with Alfacalcidol are at increased risk of hypercalcaemia. Alfacalcidol should not be co-administered with vitamin D or vitamin D derivatives due to the potential for increased side effects and increased risk. hypercalcemia. Afacalcidol may increase serum magnesium concentrations. Consider using a magnesium-free antacid. If magnesium-containing products are used with Alfacalcidol, serum magnesium levels should be closely monitored. In summary, the use of Denatri drug is to treat many diseases of osteoporosis, osteomalacia and parathyroid gland dysfunction. The drug may increase serum calcium, phosphate and magnesium. Therefore, patients should be monitored periodically for these substances during use of the drug.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.