This is an automatically translated article.
Cortifoam belongs to the group of topical steroids, indicated to support the treatment of ulcers in the rectal area. So what is the use of the drug and how is it used?
1. What is Cortifoam?
Cortifoam has the main ingredient is hydrocortisone acetate 10%, in addition to other additives propylene glycol, emulsifying wax, polyoxyethylene-10-stearyl ether, cetyl alcohol, methylparaben, propylparaben, propamine, purified water and other additives. propellant to create pressure when spraying: isobamine and propane.
Hydrocortisone acetate - is a synthetic steroid in the adrenal cortex, white powder, insoluble in water and slightly soluble in alcohol and chloroform.
Each spray contains approximately 900mg of foam including about 80mg of hydrocortisone (equivalent to 90mg of hydrocortisone acetate).
2. Indications of the drug Cortifoam
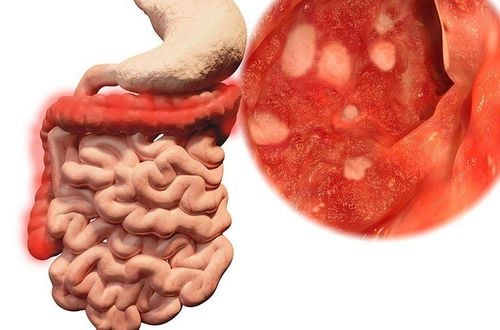
Cortifoam is indicated for the adjuvant treatment of ulcerative colitis of the distal rectum in patients unable to receive continuous oral hydrocortisone or other enema corticosteroids.However, Cortifoam is not indicated for patients who are allergic to any of the ingredients. Patients with rectal pathologies such as obstruction, abscess, rectal perforation, peritonitis, wide and deep rectal fistula, history of rectal anastomosis are also not prescribed.
3. Contraindications of Cortifoam
3.1. Dosage Use the applicator to apply to the treatment site 1-2 times in the first day. Spray 1-2 times / day, use continuously for 2-3 weeks. 3.2. How to use Shake the spray bottle vigorously for 5-10 seconds before each use. Do not remove the lid of the box while using the medicine. Hold the container upright on a flat surface and gently place your hand over the top of the spray. Pull the plunger. To fill the spray bottle, press down firmly on the side of the bottle cap, hold for 1-2 seconds and release. Pause 5 - 10 seconds to allow foam to expand in the applicator, repeat until foam reaches the full line. Remove the applicator from the lid of the container, leaving some foam on the applicator tip. Hold the spray device in place, hold the sprayer with thumb and middle finger to fix the spray, index finger on the plunger, gently insert the tip into the anus, push the plunger to push the foam out, and then withdraw the applicator. extrusion tool. Clean applicator and spray head after each use with warm water.
4. Cortifoam drug interactions
Aminoglutethimide reduces the effect of Cortifoam when used in combination. Amphotericin B injection, a drug that lowers blood potassium when combined with Cortifoam can lead to complications of cardiomegaly and congestive heart failure, synergistic hypokalemia. Macrolide antibiotics, Cholestyramine reduce the elimination of corticosteroids. Co-administration of Cortifoam with anticholinesterase agents may enhance myasthenia gravis. Concomitant administration of corticosteroids and warfarin leads to inhibition of the warfarin effect. Cortifoam can increase blood glucose levels, so the dose of antidiabetic drugs should be adjusted when used in combination. Concomitant use of Cortifoam and Cyclosporine, may increase the activity of both, increasing the risk of convulsions. Patients receiving digitalis glycoside plus Cortifoam may be at increased risk of hypokalemic arrhythmias. Estrogen, oral contraceptives, drugs that increase liver enzymes, Ketoconazole,... change the bioavailability of steroids in the blood.
5. Cortifoam side effects
Anaphylactic reactions, angioedema. Bradycardia, cardiac arrest, congestive heart failure, hypertension, hypertrophic cardiomyopathy in premature infants, myocardial rupture after recent myocardial infarction, pulmonary edema, thrombophlebitis, vasculitis. Acne, atopic dermatitis, atrophy, edema, erythema, hyperpigmentation or hypopigmentation, poor wound healing, increased sweating, rash, sterile abscess, alopecia, urticaria. Hypokalemia. Abdominal distention, increased liver enzymes, nausea, pancreatitis, peptic ulcer, hemorrhage. Osteoporosis . Emotional disturbances, convulsions. Glaucoma, cataracts.
6. Note when using the drug Cortifoam
Do not let the spray directly come into contact with the anus. The aerosol contains the medication under pressure to spray, so do not ignite the canister. Do not leave the spray bottle in a place where the temperature is higher than 50 degrees C (120 degrees F). Since Cortifoam is a direct drug that is not excreted, the systemic absorption of hydrocortisone is higher than that of other steroids. Discontinue the drug if the condition does not improve after 2-3 weeks or the disease becomes more advanced. On the heart and kidneys, the Corticosteroid component contained in Cortifoam can cause hypertension, salt and water retention, and increased excretion of potassium and calcium when used in high doses. So in a salt-restricted diet and supplemented with potassium. On the endocrine system Cortifoam may produce reversible suppression of the hypothalamic-pituitary (HPA) axis which may cause adrenal insufficiency secondary to abrupt discontinuation of the drug. The dose of corticosteroids should be adjusted in patients with thyroid disease because the drug reduces metabolism in patients with hypothyroidism and increases in patients with hyperthyroidism. Patients taking corticosteroids are likely to have reduced resistance and an increased risk of infection. Infections can range from severe to mild from any cause (virus, bacteria, fungus, parasite or helminth) in any part of the body. The higher the dose, the higher the incidence of infectious complications. Corticosteroids also mask some signs of an existing infection. Corticosteroids can aggravate systemic fungal infections, and should be used only when clearly needed. Corticosteroids are not used in cerebral malaria. Indicated in patients with latent or reactive TB disease, close monitoring is necessary because the disease may recur. Do not use live or live attenuated vaccines in patients receiving immunosuppressive doses of corticosteroids. However, certain types of immunizations may be given in patients receiving corticosteroid therapy as an alternative, eg Addison's disease. Using corticosteroids can cause cataracts, glaucoma. Do not use in patients with active ocular herpes simplex. Use with caution in patients with peptic ulcer disease because the drug increases the risk of bleeding in the gastrointestinal tract. The absolute safety of the drug on the fetus and child has not been proven, so pregnant and lactating women need to consider the benefits when using. In a nutshell, Cortifoam is a steroid in the form of an aerosol, intended for topical use in the treatment of ulcerative colitis lesions of the rectum. Medicines must be prescribed by a specialist doctor and used according to the instructions on the medicine box.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.