This is an automatically translated article.
Carmustine is an anticancer drug used to treat certain types of brain tumors; glioblastoma, brain stem glioma, astrocytoma, ependymoma,... Learn about the uses of Carmustine through the article below.
1. What are the effects of Carmustine?
Carmustine belongs to the group of anti-cancer drugs, is prepared as a yellowish lyophilized powder with a concentration of 100mg in a single-dose glass vial with a solvent vial containing 3ml of dehydrated alcohol (equivalent to 2.37g) or implant. 7.7mg. The infusion solution is diluted with physiological saline or with 5% glucose solution that is isotonic with plasma.
Carmustine alkylates RNA and DNA, has been shown by researchers to inhibit several enzymes by carbamoylation of amino acids in proteins. It is thought that the anticancer and toxic activities of carmustine may be due to metabolites.
Uses of Carmustine
Carmustine is prescribed by physicians as palliative therapy as a single agent or in established combination therapy with other proven chemotherapeutic agents The doctor prescribes the following:
Parenteral route
Brain tumors - glioblastoma, medulloblastoma, astrocytoma and metastatic brain tumors. Multiple myeloma - use in combination with glucocorticoids such as prednisone. Hodgkin's Disease and Non-Hodgkin's Lymphoma - as secondary therapy in combination with other indicated drugs in patients who relapse while on primary therapy, or who have failed to respond to primary therapy. Stable treatment prior to autologous hematopoietic stem cell transplantation (SCT) in hematologic malignancies (Hodgkin/Non-Hodgkin lymphoma) Transplant
Adult use:
Treatment as a supportive measure adjuvant surgery and radiation therapy for newly diagnosed high-grade malignant glioma. Used as an adjunct to surgery for the treatment of adult patients with multiple relapsing glioblastoma and for whom surgical resection is indicated.
2. How to use Carmustine
2.1. Dosage Adults
Intravenous route:
The recommended dose of carmustine as a single agent in previously untreated patients is 150 to 200 mg/m2 IV every 6 weeks. This medication may be given as a single injection or it may be given in a smaller dose once daily for 2 consecutive days every six weeks (such as 75 to 100 mg/m2 for two consecutive days). .
When carmustine is used in combination with other myelosuppressive medicinal products or in patients with depleted bone marrow reserves, the dosage should be adjusted accordingly.
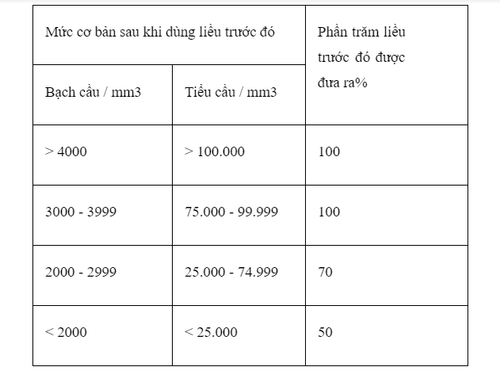
No booster injection should be given until blood components return to acceptable levels (platelets above 100,000/mm3, white blood cells above 4,000/mm3), which usually takes 6 weeks. Blood counts should be regularly monitored and repeated courses should not be given earlier than 6 weeks because of delayed-type hematologic toxicity. The next dose should be adjusted according to the patient's hematological response to the previous dose in both monotherapy as well as in combination with other myelosuppressive medicinal products. The following schedule is suggested as a guide for dosage adjustment:
Implants:
Each implant contains 7.7 mg of carmustine, creating 8 implants equivalent to a dose of 61.6 mg that are placed in the cavity where the tumor was removed.
Children
Carmustine given intravenously should be used with extreme caution in children due to the high risk of pulmonary toxicity. The safety and efficacy of the 7.7mg implant has not been established in children under 18 years of age. Other populations
Elderly people: due to the increased likelihood of impaired renal function, start with the lowest effective dose and monitor renal function regularly.
2.2. Carmustine Administration Preparation of intravenous solution
Dissolve carmustine with 3 ml of the accompanying alcohol solvent and then add the solvent for injection (27 ml) to the alcohol solution. May be further diluted to 500ml with 0.9% sodium chloride or 5% glucose infusion.
Carmustine should be administered by slow intravenous drip over a period of one to two hours. The infusion time of the reconstituted solution should not be less than one hour or it will result in burning, burning, and pain at the injection site. The injection site needs regular monitoring.
There is no time limit for carmustine infusion therapy. In cases where the tumor is still not curable despite active treatment, or because of some serious side effects or the patient's body cannot tolerate it, carmustine therapy should be discontinued.
Type of implant
Instructions for opening the package containing the implant:
Locate the folded corner and slowly pull in an outward motion. Do not rub or pull the knuckles rolling over the pack, which can put pressure on the implant and cause it to break. Remove the inner package by grasping with surgical forceps and pulling upwards. To open the inner package, gently hold it and cut in an arc around the implant. To remove the implant, gently grasp the implant with forceps and place it directly into the cavity. Up to eight implants can be placed if the size and shape of the resection drill allows. Oxidized regenerated cellulose can be used to fix the cavity surface.
3. Contraindications of Carmustine
Carmustine should not be used in people:
There is a history of previous hypersensitivity to the active substance (carmustine), to other nitrosoureas or to any of the excipients. due to previous chemotherapy or other causes. Severe renal impairment Pregnancy and lactation Children and adolescents.
4. Notes when using the drug Carmustine
Intravenous route
Pulmonary toxicity, characterized by pulmonary infiltrates and/or fibrosis, may occur within 3 years of treatment and is dose related. May cause bone marrow toxicity. Complete blood count should be monitored regularly for at least six weeks after administration. In addition, liver, kidney and lung function should be checked and monitored regularly during treatment with carmustine. This medicinal product contains 0.57 volumes of ethanol (alcohol) (7.68 g/dose). This amount of alcohol can be harmful to health, especially in alcoholics, pregnant and lactating women as well as in high-risk groups (patients with liver disease or epilepsy). when using. Appropriate contraception is required after treatment: At least 3 months (men) or 6 months (women). Implant
Care should be taken in implant-treated patients for cerebral edema/intracranial hypertension when used with steroids. CSF leaks are more common in patients treated with implants. Placement of implants adjacent to large cerebral blood vessels should be avoided. Cerebral edema with a shock effect (due to tumor recurrence, intracranial infection, or necrosis) may require reoperation and, in some cases, removal of the graft or part of it. Any passage larger than the diameter of the implant between the surgical resection space and the ventricular system should be closed prior to implantation.
5. Carmustine side effects
| Tần suất | Tiêm tĩnh mạch | Cấy ghép |
|---|---|---|
| Rất thường gặp |
- Suy tủy; khởi phát 7-14 ngày, kéo dài 21-35 ngày, hồi phục 42-56 ngày; do tích lũy, liên quan đến liều, chậm và thường hai pha. - Mất điều hòa, chóng mặt, nhức đầu. - Độc tính ở mắt, đỏ bừng kết mạc thoáng qua và mờ mắt; xuất huyết võng mạc. - Hạ huyết áp do lượng cồn từ dung môi pha (điều trị liều cao). - Viêm tĩnh mạch. - Nhiễm độc phổi, xơ hóa mô kẽ (khi điều trị kéo dài và liều tích lũy > 1400 mg / m) Viêm phổi (với liều > 450mg / m 2). - Khả năng gây nôn mửa: > 250 mg / m 2 cao; ≤250 mg / m 2 cao-trung bình. - Buồn nôn và nôn mửa, nghiêm trọng; bắt đầu trong vòng 2-4 giờ sau khi dùng và kéo dài trong 4-6 giờ. - Viêm da khi sử dụng tại chỗ |
- Động kinh: Hầu hết chúng có cường độ nhẹ đến trung bình và xảy ra trong vòng 5 ngày kể từ ngày điều trị phẫu thuật. - Chứng phù não. - Khả năng chữa lành vết thương do phẫu thuật thần kinh bị suy giảm từ nhẹ đến nặng. |
| Thường gặp |
- Thiếu máu, loạn sản tủy xương, bệnh bạch cầu cấp tính, sau khi sử dụng lâu dài. - Bệnh não (điều trị với liều cao và liều hạn chế). - Chán ăn, viêm miệng, táo bón, tiêu chảy,. - Độc tính trên gan, nhưng có thể hồi phục, thời gian chậm đến 60 ngày sau khi dùng thuốc (điều trị với liều cao và liều hạn chế), biểu hiện bằng: tăng phosphatase kiềm, bilirubin, SGOT (Glutamic-oxaloacetic transaminase) có phục hồi. - Rụng tóc - Đỏ bừng (do nồng độ cồn của dung dịch tiêm pha loãng; tăng khi thời gian dùng thuốc dưới 1-2 giờ), phản ứng tại chỗ tiêm. |
- Nhiễm trùng não (nhiễm trùng trong hộp sọ) như viêm màng não và áp xe não (có tập hợp mủ cục bộ) |
| Hiếm gặp |
- Bệnh viêm tắc tĩnh mạch (liệu pháp với liều cao). - Xơ hóa mô kẽ (với liều sử dụng thấp hơn). - Độc tính trên thận (đối với liều tích lũy < 1.000 mg / m 2). - U tuyến vú. |
|
| Không xác định tần suất |
- Nhiễm trùng cơ hội (có thể gây tử vong). - Đau cơ, co giật, trạng thái động kinh. - Đau ngực, nhịp tim nhanh,. - Nguy cơ thoát mạch: làm rộp da. - Vô sinh, quái thai. |
6. Drug interactions
Drug Interactions
The alcohol content of Carmustine may alter the effects of other drugs.
Combination with:
Phenytoin – when used concomitantly with chemotherapeutic medicinal products must be taken into account the decrease in activity of antiepileptic drugs. Cimetidine – concomitant use leads to delayed action, increased toxicity of carmustine (due to inhibition of carmustine metabolism). Digoxin – Concomitant use of carmustine may reduce the effect of digoxin (due to decreased absorption of digoxin). Melphalan – Concomitant use with Carmustine leads to an increased risk of pulmonary toxicity. Drug incompatibilities
The intravenous solution is unstable in a polyvinyl chloride container. Carmustine solution can only be stored in glass bottles or polypropylene containers.
Medicines must be used as directed and must not be mixed with other medicines.
7. How to store Carmustine
Shelf life
After reconstitution, Carmustine is stable for 24 hours under refrigerated conditions (2°C - 8°C) in a glass container.
The reconstituted solution is further diluted with 500 ml of sodium chloride for injection or 5% glucose for injection, in a glass or polypropylene container to produce a solution that can be used within 4 hours at room temperature and protected from the light. These solutions are also stable for 24 hours under refrigerated conditions (2-8°C) and for an additional 6 hours at room temperature, protected from light.
Storage
Store in the refrigerator at 2°C to 8°C.
The vial should be protected from light.
Alternatively, Carmustine can be shipped using dry ice and stored in the refrigerator at (2°C to 8°C). By following the recommended storage conditions, any decomposition or precipitation of unopened vials can be avoided until the expiration date printed on the package.
The freeze-dried product does not contain any preservatives and is suitable for single use only. There may be sharp scales in the unopened vial such as hard masses. Storing carmustine at 27°C or higher may result in liquefaction, as carmustine has a low melting point (approximately 30.5°C to 32.0°C).
The sign of decomposition is the appearance of an oily film at the bottom of the vial. This medicine should not be used any more. When it is not clear whether the product has been properly cooled, you should immediately check each vial in the carton. To verify, keep the vial in a bright light. Carmustine appears as a small amount of dried flakes or dried hard mass.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.