This is an automatically translated article.
Article written by Master, Doctor Mai Vien Phuong- Gastrointestinal endoscopist - Department of Medical Examination & Internal Medicine - Vinmec Central Park International General Hospital
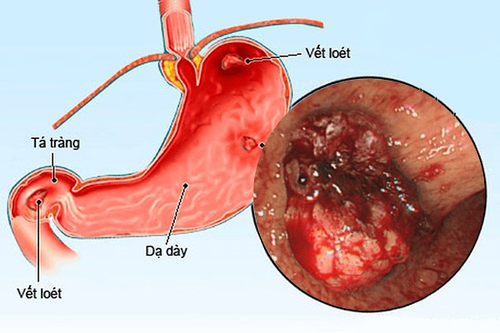
Stomach cancer is the 3rd leading cause of death in the world. There are many factors that increase the risk of this disease. Accordingly, helicobacter pylori infection was assessed as highly likely. Therefore, screening and monitoring endoscopy to detect Helicobacter pylori bacteria is essential, especially for people with a family history of stomach cancer.
1. Stomach cancer is the 3rd leading cause of death in the world
Cancer remains the third leading cause of death in the world, after cardiovascular disease and infectious diseases. In 2002, stomach cancer was the fourth most common cancer in the world, with more than 900,000 new cases and nearly two-thirds of cases occurring in developing countries. However, gastric cancer does not have a distinct geographical distribution. Indeed, countries with low rates (such as India) are also located in the region at highest risk (ie Asia). Similarly, in low-risk populations, there are also higher-risk subgroups (eg Koreans living in the US). Except for Japan, where mass screening programs have increased 5-year survival rates by about 60%, in most other parts of the world only 20% of gastric cancer patients are still alive. after 5 years. Mortality is influenced by several factors: cancer incidence, stage of disease at diagnosis, biological factors, and individual response to current treatment. Therefore, any intervention aimed at reducing cancer incidence (i.e. eliminating/modifying potential risk factors and/or promoting protective factors), early diagnosis (i.e. identifying and monitoring patients at increased risk) or improve access to health care, will help reduce cancer mortality. Risk factors for stomach cancer.
Several risk factors have been identified for extra-cardiac gastric cancer such as: Helicobacter pylori infection, low socioeconomic status, smoking, consumption of salted and smoked foods, and low consumption of vegetables and fruits. and a family history of stomach cancer.
Trắc nghiệm: Bạn có biết những sự thật này về dạ dày không?
Hoạt động của dạ dày là một hoạt động quan trọng giúp cơ thể dung nạp và chuyển hóa dinh dưỡng từ thực phẩm hàng ngày. Tuy nhiên, không phải ai cũng biết rõ về dạ dày cũng như các vấn đề xoay quanh hoạt động tiêu hóa thức ăn của nó. Hãy cùng tìm hiểu kỹ hơn trong bài trắc nghiệm dưới đây.
Bài dịch từ: webmd.com
2. When should an H. pylori infection be treated?
The prevalence of H. pylori infection varies from country to country, from stage to stage, and the indications for treatment also vary from period to period. According to the consensus of gastroenterology conferences in Vietnam and around the world for many years, the indication for H. pylori eradication treatment is considered necessary for children and adults with peptic ulcer disease with H. . pylori positive and for H. pylori positive people who have a family history of stomach cancer. During the 2015 Asia-Pacific Digestive Diseases Week conference in Taiwan, several authors suggested eradication therapy for all members of the family with a person infected with H. pylori even without symptoms. evidence, in order to avoid H. pylori infection through food, this is an issue that needs further discussion. Successful treatment of H. pylori eradication, according to world studies, notably studies in Japan and China from more than 10 years ago, shows a significant reduction in the rate of gastric cancer compared with those who have been treated for eradication but have failed. Thus, prevention and eradication of H. pylori can reduce the risk of disease in children, including stomach cancer in adulthood? This is an issue that needs to be studied and followed up for a long time to elucidate further.
3. Family history and genetic syndromes
About 10% of gastric cancer patients have a family member with this disease and hereditary gastric cancer accounts for only 1%-3% of cases [Yaghoobi M et al., 2010; Palli D et al., 1994]. Many studies report a 1.5 to 3.5-fold increase in the risk of developing the disease in first-degree relatives of patients with gastric cancer [Dicken BJ et al., 2005]. Furthermore, in the study by Brenner et al. published in 2000, a family history of gastric cancer showed a relative risk of 2.9 (95% CI: 1.3-6.5) and disease development is also associated with an increased prevalence of H. pylori infection. It is noteworthy that the presence of both factors (family history of stomach cancer and H. pylori infection) increased the risk of developing the disease eightfold. Stomach cancer has sometimes been described as part of a number of inherited cancer syndromes such as familial adenomatous polyposis, Peutz-Jeghers syndrome, and familial nonpolyposis colon cancer syndrome. In 1998, Guilford et al reported the first cases of hereditary disseminated gastric cancer (HDGC) [Caldas C et al., 1999]. The authors present three families with multigenerational early-onset diffuse gastric cancer. At the molecular level, gamete mutations in the E-cadherin (CDH1) gene were found in these families. Therefore, in families with at least two people with diffuse gastric cancer and at least one early-onset case (diagnosed before age 50), mutation testing is recommended [Brooks-Wilson AR et al., 2004]. Finally, in subjects carrying harmful CDH1 gene mutations, active endoscopic monitoring is not warranted and the currently recommended curative treatment is prophylactic gastrectomy to avoid the develop this deadly disease.

4. Treatment of Helicobacter pylori and the risk of stomach cancer
H. pylori infection is clearly correlated with gastric carcinogenesis. The strongest evidence supporting an association between H. pylori infection and gastric cancer development comes from prospective cohort studies. A meta-analysis of data from 12 prospective cohort studies showed a 6-fold increased risk after 10 years of follow-up. However, it remains uncertain whether eradication of H. pylori is an effective chemopreventive strategy to reduce gastric cancer risk. In particular, there are only two interventional, randomized, controlled studies [Wong BC et al., 2004; Fukase K et al., 2008] in which gastric cancer development was the primary outcome measure. These two studies were conducted in regions with a high risk of stomach cancer, China and Japan. However, the two studies had different designs. Wong et al.'s study was a placebo-controlled study and enrolled patients with or without precancerous lesions of the stomach (eg, atrophy, dysplasia, or intestinal dysplasia), but patients with gastric cancer. early stomach was excluded (Table 2). In contrast, Fukase et al.'s study was an open study, enrolling patients with early gastric cancer, newly diagnosed or scheduled for endoscopic treatment or being monitored after gastrectomy. In Wong et al.'s study [2004], 1630 patients with H. pylori infection were enrolled and during 7 years of follow-up, 7 out of 817 (0.9%) subjects were in the eradication group and 11 out of 813 (1.3%) of the placebo group subjects developed gastric cancer (P=0.33). However, the most interesting finding comes from a post hoc analysis. In the active drug group, none of the patients on baseline histology showing no precancerous lesions developed cancer. In contrast, all patients who developed gastric cancer had precancerous lesions when enrolled in the study. Wong et al concluded that prophylactic bacteriostatic treatment is effective only in the early stages of carcinogenesis, before the development of precancerous lesions, and they suggest a " point of no return in the chain of events leading to stomach cancer. In a multicenter study, Fukase et al. [2008] enrolled 544 patients and after 3 years of follow-up found 9 out of 272 patients in the eradication group and 24 out of 272 patients in the eradication group. Untreated stomach cancer. Bacterial eradication significantly reduced the risk of developing cancer after endoscopic therapy, with OR = 0.353 (95% CI: 0.161-0.775, P = 0.009). This study provides evidence of a prophylactic effect of H. pylori eradication treatment even in later stages of carcinogenesis, in stark contrast to the conclusions of Wong et al. There are several possible reasons for this difference: study design, sample size, endoscopic evaluation technique, histological definition, and duration of follow-up.

In the future, routine use of the new histological staging system is proposed (eg OLGA) [Fuccio L et al., 2008; Fuccio L et al., 2007] and better identification of host and microbial risk factors may help to more accurately classify patients requiring endoscopic follow-up. Although the incidence of stomach cancer is decreasing, it is too early to say that it is a rare cancer and the mortality rate worldwide remains high. Primary prevention is a viable strategy because most risk factors can be eliminated.
However, gastric cancer is a multifactorial disease, so addressing one factor (eg, H. pylori infection) does not prevent all cases. Endoscopic follow-up is still necessary, especially in high-risk subjects. Although the definition of high-risk patient is not well defined, it is reasonable to consider patients with a family history of gastric cancer, patients with precancerous lesions, and patients with a diagnosis of cancer. pre-existing stomach is at high risk. All manageable risk factors, such as smoking cessation, high fruit and vegetable intake, and H. pylori eradication therapy, should be considered as preventive strategies. Defining high-risk patients, as well as finding the best surveillance strategy for those patients. challenge in the future.
Vinmec International General Hospital is a prestigious address trusted by many patients in performing diagnostic and treatment techniques for gastritis and duodenal diseases... Along with that, at Vinmec, the practice Currently diagnosed through gastroduodenal endoscopy with Olympus CV 190 endoscope, with NBI function (Narrow Banding Imaging - endoscope with narrow light frequency band) for clear mucosal pathological analysis results than conventional endoscopy, detecting ulcerative lesions in the gastrointestinal tract. Vinmec International General Hospital with modern facilities and equipment and a team of experienced specialists who are always dedicated in medical examination and treatment, customers can be assured of colorectal endoscopy service. colon here.
Customers who are interested in digestive disease diagnosis services and want to screen and detect early gastric cancer since they have no symptoms can contact Vinmec International General Hospital for advice. support.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.