This is an automatically translated article.
Posted by Master, Doctor Mai Vien Phuong - Gastrointestinal Endoscopy - Department of Medical Examination & Internal Medicine - Vinmec Central Park International General Hospital.RON receptor tyrosine kinase (RTK; also known as MST1R) was first identified in 1993 in a cDNA library from human epithelial cells. RON belongs to the c-MET family of proto-oncogenes. This RTK family has only two members, RON and Met, with only 34% overall similarity. However, the tyrosine kinase regions of the receptors were quite similar at 80% similarity. In 1994, a mouse cDNA was cloned encoding a homolog of RON, called the stem cell-derived tyrosine kinase receptor.
1. RON . activation and signaling pathway mechanisms
Epithelial cells in the skin, adrenal glands, bones, brain, kidneys, intestines, lungs and liver express low levels of RON. RON activity plays an important role in epithelial cell motility, enhancement of adhesion, sperm motility in the epididymis and embryonic development, as well as regulation of spermatogenesis. inflammatory reactions. Under physiological conditions, the primary cause of RON activation is stimulation of its ligand, the macrophage-stimulating protein.
2. Different biochemical processes activate RON in tumors
Three other biochemical events trigger RON in the tumor: RON overexpression, induction of oncogenic RON variants, and RON metabolism. The RON receptor consists of three essential regions: an extracellular domain that receives its ligand, a transmembrane domain that holds the receptor to the membrane, and an intracellular domain that performs kinase activity. The first step for RON activation is cell-surface dimerization, which is caused by the binding of a macrophage-stimulating protein to an extracellular region containing a specific ligand-binding site, potentially leads to a structural change in the RON receptor.
This activation leads to autophosphorylation of two tyrosine residues (Tyr1238 and Tyr1239) located in the A-ring (Phe1227-Pro1250) of the kinase domain. The phosphorylation of these regulatory moieties leads to the activation of tyrosine kinase function, which induces further phosphorylation of the Tyr1353 and Tyr1360 residues located at the C-terminal binding site. This then acquires the molecules. cytoplasmic receptor-binding protein 2 (GRB2) and Son of Sevenless in cytoplasmic molecules. In addition, the ubiquitin ligase, B-lineage lymphoma (CBL), binds to the binding site to act as a negative modulator.
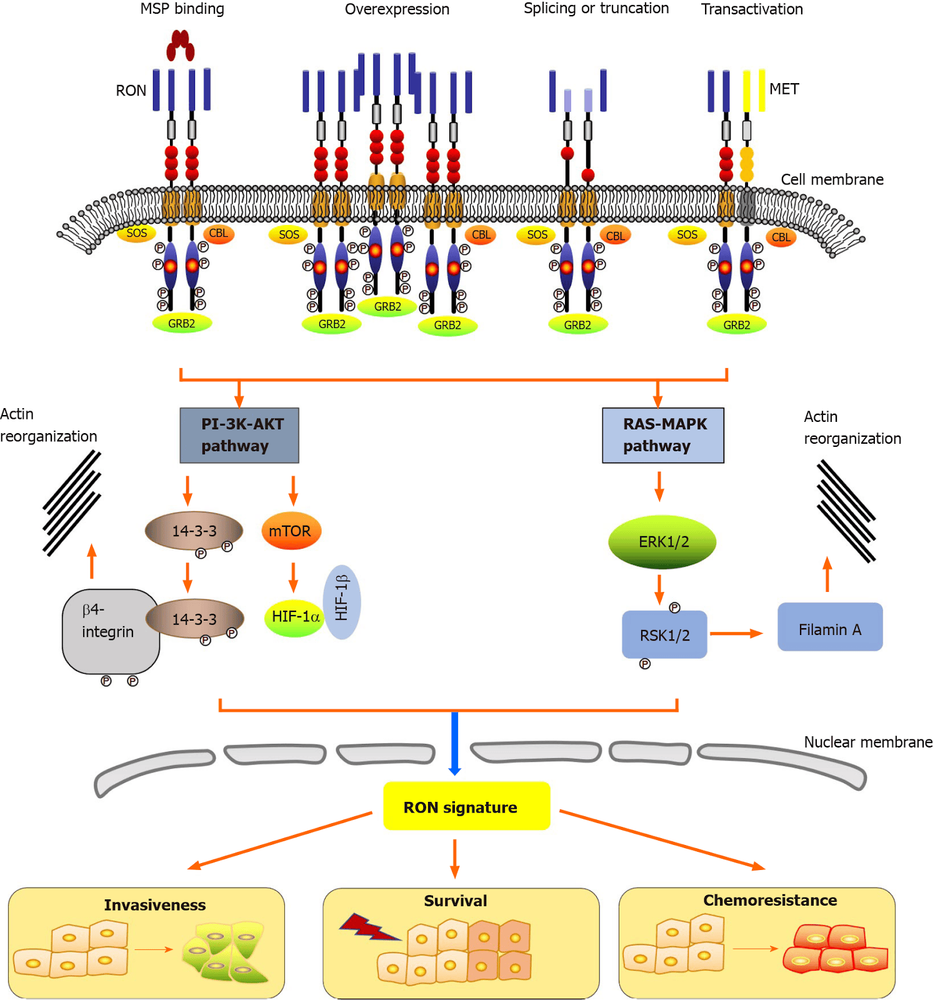
3. RON activation mechanism and downstream signaling pathways
Classically, macrophage-stimulating protein (macrophage-stimulating protein) activates RON. In cancer, RON activation is induced by overexpression, splicing or truncation, and metabolism. The RON receptor consists of three regions including an extracellular domain, a transmembrane domain, as well as an intracellular domain. The macrophage-stimulating protein binds to the extracellular domain leading to autophosphorylation of several tyrosine residues in the kinase activation loop or at the C-terminal tail, resulting in the activation of many biological activities, including proliferation/ survival, cellular invasive activity and chemical resistance. Macrophage Stimulating Protein; SOS: Son of Sevenless; GRB2: Growth factor receptor 2 binding protein; CBL: Casitas B-line lymphoma; 14-3-3: Tyrosine 3-monooxygenase / tryptophan 5-monooxygenase activated protein; PI-3K-AKT:Phosphatidylinositol-4,5-Bisphosphate 3 kinase- protein kinase B; HIF: Hypoxia-inducible factor; RAS-MAPK: RAS-mitogen-activated protein kinase; ERK: Extracellular Regulatory Kinase; RSK: Ribosomal protein S6 kinase; mTOR: Mechanistic target of rapamycin.
4. RON's interaction with synaptic proteins represents the first step in the bridging of downstream signaling cascades and RON activation
The interaction of RON with synaptic proteins, such as β-arrestin-1 and GRB2, represents the first step in the bridging of downstream signaling cascades and RON activation. Through its C-terminal binding site, RON interacts with many cytoplasmic effector molecules, such as phospholipase C gamma, phosphatidylinositol-4,5-Bisphosphate 3-kinase (PI-3 kinase), Src( SRC proto-oncogene, non-tyrosine kinase receptor), tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activated protein (14-3-3), CBL protooncogene (c-Cbl), heat shock protein family A (Hsp70) member 8 (HSC70), Integral-β4, plectin, and protein phosphatase 1. The classical PI-3 kinase-protein kinase B (PI-3K-AKT) and RAS-mitogen-activated protein kinase (RAS-MAPK) pathways are identified. activated by the interaction of the binding site of RON with downstream signaling proteins. The PI-3K-AKT and RAS-MAPK pathways mediate many biological activities, including proliferation and survival, EMT, cellular invasive activity, and chemoresistance. RON signaling pathways are also play a part in regulating tumor activity. Among them, coordinated activation of the PI-3K-AKT and RAS-MAPK pathways plays an important role in EMT through increased cell motility. Studies using an MDCK cell model show that RON-mediated EMT is associated with reduced E-Cadherin expression and uncontrolled vimentin expression, mediated by RAS-MAPK signaling. The main protein that binds EMT to RON signaling is the ribosomal protein S6 kinase-2, which is an mediator in the MAPK pathway. RON-mediated PI-3K-AKT signaling is also implicated in invasive development, including increased epithelial cell substrate invasion, in vitro migration and adhesion, and distant metastasis and tumor cell invasion in vivo5. RON receptor protein sign and expression in cancer patients
In general, normal epithelial cells, including cells from the colon, lung, and breast, express low levels of RON. However, cells of mesenchymal origin do not express RON. RON activation in tumors is often the result of receptor overexpression, as opposed to classical macrophage-stimulating protein binding. Unregulated RON activation and expression are detected in a variety of cancers and have prognostic implications for patient survival. Results from the majority of published studies suggest that dysregulation of RON expression is characterized primarily by increased expression of wild-type RON and production of active isoforms, ultimately leading to persistent activation of downstream signaling cascades. There have been reports of RON amplification and point mutations. However, this type of genetic variation is rarely observed. The relationship between carcinogenesis and dysregulated RON expression and signaling has been demonstrated through functional studies using immunohistochemistry (IHC) staining of the specimens. tumors and cancer cell lines.
6. Overexpression of RON in cancer tissue
The first report of wild-type RON overexpression in cancer tissue was in primary breast cancer samples. Subsequently, IHC staining further detected wild-type RON in thyroid, bladder, adrenal gland, head and neck, uterus, skin, lung, kidney, pancreas, colorectal and other tumors. These findings are consistent with results found in other cancers, such as human glioma, melanoma, and Merkel cell carcinoma, which suggest that RON expression is not normal It has also been implicated in both neuroblastoma and skin cancer. In breast tissues, expression of RON is relatively low in normal breast epithelial cells and even in cells from benign lesions (papillomas and adenomas), whereas it is highly expressed. in 47% (35/75 cases) of histologically altered tumor samples.7. Dysregulated expression of RON functions in human breast carcinomas progressing to metastatic invasive phenotype
RON upregulation is closely associated with its phosphorylation status and invasive activity, suggesting that the dysregulated expression of RON functions in human breast carcinomas progressing to breast cancer. invasive-metastatic phenotype. Furthermore, in breast cancer uncontrolled RON expression was identified as an independent predictor of distant recurrence. In contrast, in certain tumors, such as hepatocellular carcinoma (HCC), the frequency of wild-type RON expression is relatively low. However, its significance remains unknown, although this finding indicates that wild-type RON is not universally expressed in different tumor types. Furthermore, overexpression of RON is associated with the production of oncogenic RON isoforms, e.g. RON 160, including deletions of exons 5 and 6, which encode 109 amino acids in the extracellular sequence. RON β. RON variants are detected in primary cancer samples and cell lines relatively frequently and are detected in 40% to 60% of cases. The carcinogenesis mechanism and clinical relevance may be influenced by the frequency and extent of the RON isoform.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
REFERENCES
Chen SL, Wang GP, Shi DR, Yao SH, Chen KD, Yao HP. RON in hepatobiliary and pancreatic cancers: Pathogenesis and potential therapeutic targets. World J Gastroenterol 2021; 27(20): 2507-2520 [DOI: 10.3748/wjg.v27.i20.2507]