This is an automatically translated article.
Post by Master, Doctor Mai Vien Phuong - Department of Examination & Internal Medicine - Vinmec Central Park International General Hospital
The cause of hepatitis B infection in young children is mainly because the disease is passed from mother to child. In fact, in Asian countries, this is a common route of hepatitis B infection because people's knowledge about this disease is limited, many people are not fully vaccinated. In Vietnam, too, many women are not screened before and during pregnancy. According to some studies, in Vietnam, more than 10% of pregnant women are infected with hepatitis B virus. This is a very large percentage.
1. Current Pediatric Guidelines for the Treatment of Children with HBV
Current pediatric guidelines recommend limiting treatment to children with persistent (>6 months) active hepatitis B and evidence of fibrosis, and observing those with an immune-tolerant pattern. The rationale for this approach is that HBeAg-positive CHB, especially when it is prolonged and started early, at age <3, has the highest risk of progression to cirrhosis, ranging from 1% to 5% by third decade.
Many centers tend to delay treatment based on the correct notion that CHB is harmless and that transaminase activity heralds spontaneous immune clearance. Furthermore, there is evidence that treatment only increases the rate of HBeAg/HBeAb seroconversion without affecting the proportion of patients with seroconversion over time. On the other hand, treatment may be indicated to break tolerance. The EASL recommends nucleoside or nucleotide analogues (NAs) for long-term viral suppression in immune-tolerant patients 30 years of age or older, to reduce the risk of fibrosis and cirrhosis in those with delayed immune clearance. In fact, studies highlight that high viral load in persistently positive HBeAg patients is associated with the greatest risk of cirrhosis, HCC and liver-related death and infection control HBeAg/HBeAb seroconversion function reduces those risks. Therefore, the question has arisen whether treatment should be given to children with immunity.
Childhood age considered appropriate time to break viral tolerance
The first encouraging experience with the treatment of immunocompromised children was published in 2006. Clearance HBV-DNA and HBsAg/HBsAb seroconversion were achieved in 5 of 22 infants (23%) after 10 months of sequential combination therapy with lamivudine and IFN-a. That result was confirmed in a case-control study in which 6 of 22 immunocompetent infants achieved HBsAb-dependent functional control. Another study reported a success rate of 33%. Those studies reported HBsAb seroconversion rates of 20%-25%. In contrast, a clinical trial using the combination of entecavir and peg-IFN-a 2a resulted in only 2 of 60 children achieving the primary endpoint of HBeAg loss and a sustained HBV-DNA level <1000 IU/mL.) , with adverse events reported in more than 50% of children
2. Drugs approved for children with chronic HBV infection
Seven different drugs have been approved by the US FDA and EMA for children and adolescents with chronic HBV infection. IFN- and peg-IFN- are immunomodulators that can be administered for a predetermined period with the aim of inducing immune-mediated control of HBV infection and achieving long-term suppression. viral replication after treatment. NAs are potent HBV inhibitors used as long-term oral treatments to suppress viral replication, or as finite-duration treatments with or without IFN, to achieve sustained virological response after treatment. NAs are also classified as a genetic barrier to resistance according to the threshold of mutations required for clinically significant loss of susceptibility. Lamivudine, adefovir, and telbivudine have low tenofovir and tenofovir, and entecavir has a high genetic barrier to resistance. Treatment is indicated for children and adolescents with active viral replication (detectable HBV-DNA levels), persistent active hepatitis (6-12 months) (transaminase levels in serum elevation) and/or inflammation or fibrosis on liver biopsy. Unless in a clinical trial, treatment is contraindicated when transaminases are normal. Antiretroviral drugs are approved for adolescents and children with chronic hepatitis B virus infection.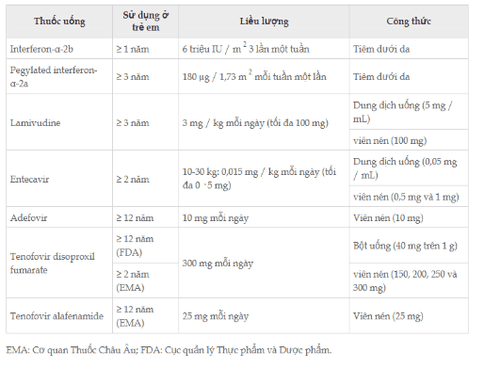
IFN - 2b, peg-IFN - 2a, lamivudine, adefovir, tenofovir disoproxil fumarate, and entecavir have been approved for the treatment of children and adolescents with chronic HBV infection following six randomized controlled trials with placebo. Tenofovir alafenamide was approved on the basis of studies of its use in HIV infection (Table 4). IFN therapy may be associated with a higher rate of HBsAg loss compared with NA. In all studies, an overall good treatment response was defined by reduction of serum HBV-DNA to undetectable levels, loss of serum HBeAg, and normalization of transaminases, which were associated and elevated scores. baseline histological activity index, increased baseline transaminase levels and decreased baseline HBV-DNA levels. Peg-IFN, entecavir, and tenofovir disoproxil fumarate are considered the drugs of choice for the treatment of CHB in children by major international societies. The advantage of using IFNs and peg-IFNs in children, compared with NAs, is the absence of viral resistance and the predictable, finite duration of treatment. However, the use of IFN and peg-IFN is required in children because it requires subcutaneous injection and is associated with the almost certain occurrence of flu-like symptoms.
Conclusion
For HBV infection, recent cohort studies have clarified that several factors including immune host phenotype, viral genotype and ethnicity, contribute to spontaneous control. Viral hepatitis should not be a barrier to the use of immunosuppressants in the treatment of autoimmune diseases, as well as anticancer chemotherapy, as long as timely screening and interventions are implemented. appropriate pharmacology. Finally, new drugs are being developed to treat HBV, which primarily work by promoting tolerance breakdown.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.