This is an automatically translated article.
Post by Master, Doctor Mai Vien Phuong - Department of Examination & Internal Medicine - Vinmec Central Park International General Hospital
Existing drugs against HBV have inherent limitations. NA is safe and well-tolerated and is often successful in blocking replication. They have no curative effect, as they act late in the viral cycle and do not prevent HBV-DNA persistence in an integrated or episodic form.
1. Overview
IFNs have limited efficacy and frequent side effects, but they are more likely to achieve certain “functional” treatment with loss of HBeAg or HBsAg, seroconversion, and normal and undetectable transaminases. viruses). Various new compounds are being investigated with the aim of achieving high HBsAg seroconversion rates. The candidate drugs are relevant in the field of pediatrics, as almost all of them act as immune modifiers to achieve tolerability breakthroughs. The molecules are currently in phase II and further trials are listed and summarized in Table 1
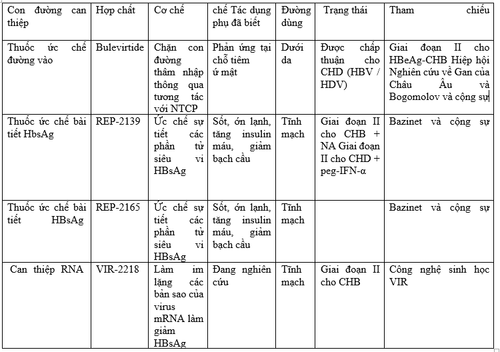
2. Drugs being studied in the treatment of HBV in children
Capsid Aggregation Inhibitors Capsid Aggregation Inhibitors are complementary antiviral agents for NA. JNJ-56136379 is an oral compound that has two distinct mechanisms, inhibiting the packaging of pre-genomic RNA (pgRNA) and the formation of covalent closed-loop DNA (ccc). It was studied in 57 subjects with CHB treated for 28 days. HBV-DNA and HBV-RNA decreased at all doses tested and HBV-DNA was undetectable at the end of the study in one-third of the patients. However, none of the cases achieved HBsAg/HBsAb seroconversion. ABI-H0731 (Vebicorvir) is an oral compound that inhibits encapsulation, binding to core proteins, and thus prevents pgRNA packaging into nucleocapsids. A phase I study performed in healthy volunteers and 38 CHB patients reported good tolerability and a rapid but temporary reduction of virus in the blood. Two phase II studies are underway, with interim results showing that in patients with already suppressed NA, the addition of Vebicorvir significantly suppressed HBV-RNA levels. In treatment-nave patients, its combination with standard of care resulted in greater reductions in HBV DNA levels Nucleic acid polymerase inhibitors Nucleic acid molecules reduce the release of HBsAg small viral particles, which are considered crucial in immune system depletion, thereby favoring HBsAg loss and seroconversion to HBsAb. Polymer REP-2139, administered intravenously once weekly, was selected for its tolerability in this group. Sequential administration of REP-2139 and peg-IFN-a in chronic HBV/HDV co-infection over a 63-week period, resulted in sustained loss of HBsAg and seroconversion to HBsAb in six of 12 patients. HBV-DNA and HDV-RNA were negative in seven patients at the end of treatment and nine at 1-year follow-up. In HBeAg-negative CHB, REP-2139 or its analogue REP-2165 was used in combination with tenofovir and peg-IFN-a and sustained HBsAg/HBsAb seroconversion was achieved in 41% of patients and functional control (undetectable HBV-DNA and normal transaminase) in 77%, an unprecedented result RNA Interference Drug
RNA interference is another promising strategy aimed at silencing too viral translation and subsequently reduce HBsAg. Preliminary efficacy reports are available from ongoing phase I/II studies on CHB involving the administration of 3 moly small interfering RNA (si) JNJ-3989 (ARO-HBV). Regardless of HBeAg status and prior treatment, HBsAg decreased by 97%-100% after a single dose, and the majority of participants achieved HBsAg loss and significant reduction in HBV-DNA immediately upon completion of the regimen. The drug is aimed at disrupting the immune suppression to the virus, as suggested by an ongoing trial in immune-tolerant adults. Similar interim results were reported in a trial of siRNA VIR-2218 Immunomodulators Immunomodulators are a heterogeneous class of candidate antiviral drugs that target other agents. of innate immunity. Inarigivir is a pattern-recognition receptor agonist RIG-1, whose agonist activates the IFN-I response. Final results of the phase II ACHIEVE trial demonstrated a dose-dependent decrease in HBV-DNA following inarigivir monotherapy, and an endpoint of HBsAg reduction >0.5 log 10 was achieved in 22% patient. Selgantolimod (formerly GS-9688), is a potent, orally administered Toll-like-receptor (TLR8) agonist capable of inducing tumor necrosis factor-, IFN-γ, interleukin ( IL)-12, and IL-18 expression. Interim results of the phase II study showed that it significantly reduced HBsAg in 16%-30% of CHB patients and sometimes lost HBsAg 24 weeks after initiating treatment. Bulevirtide is the only viral entry inhibitor approved by the EMA in 2020 for HBV/HDV coinfection, while it is in a phase II study for HBeAg-negative CHB (NCT02881008). It binds to sodium taurocholate cotransporting polypeptide (NTCP) to prevent HBV from entering hepatocytes. In combination with peg-IFN-a, it was shown to significantly reduce HBV-DNA and HDV-DNA compared with peg-IFN-a alone. Figure 1 depicts the different mechanisms of action of HBV investigational products. Although there are still many steps to achieving availability in the pediatric population, these drugs will certainly change the burden of HBV in children as much as in adults. The view of possible treatments and cures aimed at breaking tolerance will increase efforts to eliminate HBV early in life.
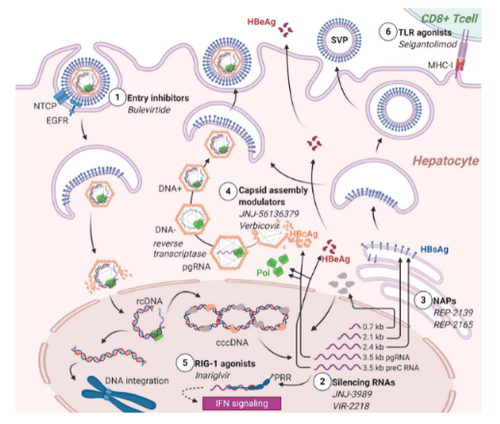
New research approaches to chronic hepatitis . cccDNA: Covalent closed loop DNA; DNA-: Synthetic DNA strand negative; DNA +: Synthetic positive sense strand DNA; EGFR: Epidermal growth factor receptor; NTCP: Sodium taurocholate cotransporting polypeptide; HBcAg: Hepatitis B Core Antigen; HBeAg: Hepatitis B e-antigen; HBsAg: Hepatitis B Surface Antigen; NAPs: Nucleic Acid Polymers; pgRNA: protogenomic RNA; preC RNA: precore RNA; PRR: Pattern Recognition Authority; rcDNA: dilated circular DNA; SVP: Small virus particles; TLR: Cell phone-like receptor.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.