This is an automatically translated article.
The article was written by MSc.Nguyen Van Office - Cell Therapy Block, Vinmec High-Tech CenterImmunotherapy is being considered as an advanced cancer treatment method, increasing the effectiveness of treatment when combined with traditional methods (surgery, chemotherapy and radiation). We often think of that immunotherapy as a medical achievement that has only begun in the last few years. However, the fact that immunotherapy in cancer treatment has existed for a long time under many different names or descriptions throughout history.
1. General introduction
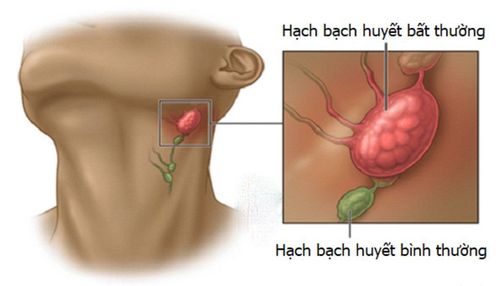
Cancer is a large group of diseases that involve abnormal cell proliferation with the ability to invade and metastasize to other parts of the body. Currently, cancer is the second leading cause of death globally, according to WHO statistics in 2018 there were 9.6 million deaths from cancer.In cancer treatment, surgery, chemotherapy and radiation therapy are considered traditional cancer treatment methods that have brought effective results to patients. In addition to traditional cancer treatments, there are many new cancer treatments that promise effective treatment and improve patients' quality of life.
Trắc nghiệm: Thử hiểu biết của bạn về bệnh ung thư
Ung thư là nguyên nhân gây tử vong hàng thứ 2 trên thế giới. Thử sức cùng bài trắc nghiệm sau đây sẽ giúp bạn có thêm kiến thức về yếu tố nguy cơ cũng như cách phòng ngừa bệnh ung thư.
Bài dịch từ: webmd.com
2. History and development of immunotherapy
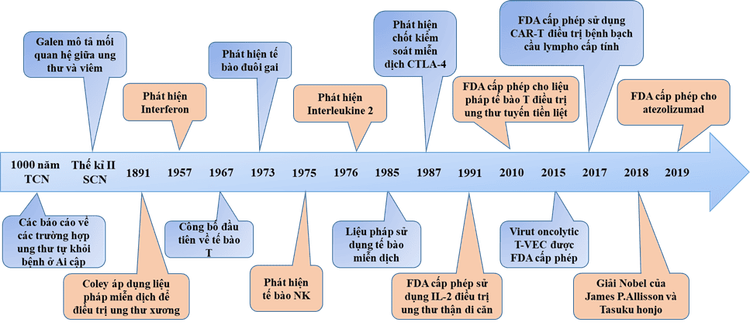
From the time of Ancient Egypt, about 3,000 years ago until the beginning of the 19th century, there were many reports and anecdotes of tumors disappearing spontaneously or after infection accompanied by high fever. In the 2nd century AD, Claudius Galenus first described the correlation between cancer and the inflammatory process.In 1866, Fehleisen and Busch noticed a decrease in tumor size after a cancer patient developed erysipelas. In 1883, Fehleisen identified the strain of bacteria causing erysipelas and shrinking tumor size as Streptococcus pyogenes.
The next important advances came from William Bradley Coley, who is now known as the father of immunotherapy. Coley first manipulated the immune system to treat bone cancer in 1891. He directly observed a number of cases where cancer patients went into remission after having erysipelas. Coley also researched medical records and medical literature he had access to in the late 19th century and discovered 47 case reports of patients with potentially incurable cancers. remission after concurrent acute infection.
From 1891, Coley began injecting different mixtures of active Streptococcus pyogenes, inactivated Streptococcus pyogenes and Serratia marcescens into the patient's tumor, thus being the first to develop a treatment method. Immune-based cancer treatment. Coley has achieved successful clinical outcomes in the treatment of sarcoma, lymphoma and testicular carcinoma, in more than 1,000 cases of tumor reduction or complete cure.
Research on the immune system returned to interest after 1945, with many advances in immunology and cancer research. In 1967, Jacques Miller described the function of T cells. In 1973, Steinman discovered dendritic cells, followed by natural killer cells (NK cells) in 1975 by Klein. Around the same time, researchers and doctors from the University of Minnesota pioneered bone marrow transplants to treat blood cancers. In the 1980s, after a series of new discoveries, the field of immunotherapy finally made a real comeback.
During 40 years of development to this day, a series of important research and test results are published. Various treatments based on the activity of the immune system have achieved great success in both animal and clinical models, promising immunotherapy as the fourth cancer therapy along with cancer. surgery, chemotherapy and radiation therapy.
3. Typical immunotherapies used in cancer treatment
3.1 Antibody therapy Monoclonal antibody therapy has been around since the FDA approved muromonab-CD3 in 1986, and the advent of trastuzumab and rituximab in the 1990s revolutionized cancer treatment. To date, more than 30 monoclonal antibodies have been licensed for a variety of therapeutic indications.Tositumomab was licensed for the treatment of non-Hodgkin lymphoma in 2003. This was followed by bevacizumab and cetuximab in 2004 for the treatment of metastatic colorectal cancer.
In 2010, the targeted antibody brentuximab was licensed for the treatment of relapsed, refractory Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Obinutuzumab was licensed for the treatment of chronic lymphocytic leukemia in 2013 and follicular lymphoma in 2016.
3.2 Interleukine-2 (IL-2), Interferon (IFN) and other cytokines IFN-α is The first cytokine was discovered in 1957 by Isaacs and Lindenmann. IL-2 was discovered in 1976, allowing scientists to grow T lymphocytes in vitro.
In 1984, IL-2 was used to treat cancer in a 33-year-old patient with metastatic melanoma. Through successful clinical studies and results, IL-2 has been shown to be effective when used in high doses in patients with metastatic cancer through enhancing T-lymphocyte production.
In 1991, the FDA approved the use of IL-2 for the treatment of metastatic kidney cancer and for the treatment of metastatic melanoma in 1998. From the 2000s to the present, cytokine therapies are still being developed. strongly developed and proven effective when used in combination with other methods.
3.3 Cancer vaccines Development and testing of vaccines to prevent cancer caused by the HPV virus was developed in the early 2000s, the Gardasil vaccine was the first successful vaccine approved by the FDA. approved in 2006. In 2010 an autologous cancer vaccine using dendritic cells was approved by the FDA for the treatment of prostate cancer.
In 2014, GVAX Pancreas and the cancer vaccine CRS-207 were shown to work in patients with pancreatic cancer in a phase 2 trial. In 2015, Talimogene laherparepvec was approved by the FDA for subcutaneous administration. skin in melanoma patients. Today, cancer vaccines are focused on research and development by scientists, promising many new opportunities for cancer patients in the future.

Other oncolytic viruses such as Pexa-Vec (treatment of hepatocellular carcinoma), CG0070 (treatment of bladder cancer), G471 (treatment of glioblastoma and prostate cancer) and one Others have been shown to be highly effective in clinical trials.
Although many successful results have been achieved, the disadvantage of using oncolytic viruses is the ability of the body's immune response, the immune system will fight the oncolytic virus when re-treatment.
3.5 Immune checkpoint inhibitor therapy The discovery of immune checkpoints such as CTLA-4 (cytotoxic T lymphocyte-associated antigen 4) and PD-1 (Program death 1) has contributed to the development of Checkpoint inhibitors for cancer treatment.
In 2002, the first clinical trials of a monoclonal antibody to inhibit CTLA-4 were conducted in kidney and prostate cancer. In 2011, the FDA approved ipilimumab for the treatment of melanoma. In 2013, nivolumab was licensed for the treatment of relapsed/refractory classical Hodgkin lymphoma following stem cell transplantation and brentuximab. In 2014, pembrolizumab (Keytruda), a PD-1 inhibitor, was rapidly approved for the treatment of advanced or unresectable melanoma. In 2015, the melanoma therapy, which combines ipilimumab with nivolumab, was approved by the FDA. That same year, nivolumab was approved by the FDA for the treatment of advanced renal cell carcinoma. 3.6 Immune cell culture and proliferation therapy In the early 2000s, T cells have been studied for various therapeutic purposes including:
(1) direct use of T cells to treat cancer cancer (2002 melanoma)
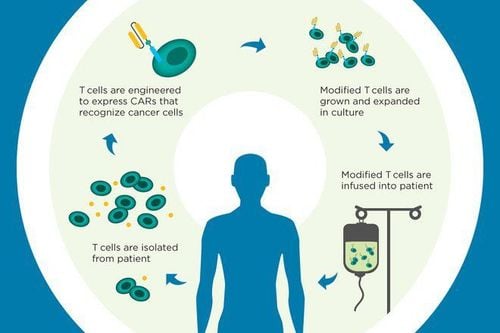
(2) using genetically modified T cells (CAR-T cells)
CAR-T cells have responded to patients with B-cell lymphoma and lymphoma chronic. In 2013, clinical trials using CAR-T cell therapy produced amazing results, complete responses in patients with B-cell acute lymphoblastic leukemia, 89% in children, and 89% in children. in adults with acute lymphoblastic leukemia and 92% in patients with non-Hodgkin lymphoma. In 2017, CAR-T cell therapy was approved for the treatment of children with acute lymphoblastic leukemia, becoming the first FDA-approved treatment. In addition to T-cell therapy, NK-cell therapy has also achieved great success in cancer treatment.
Currently, Vinmec International General Hospital is one of the first facilities in Vietnam to receive technology transfer and application of autologous immune-boosting therapy to support the treatment of cancer patients from the National Institute of Cancer Medicine. Biotherapy Institute of Japan (BIJ).
Autologous immunosuppressive therapy using a combination of both cytotoxic NK and T cell lines to treat solid cancer patients has obtained positive results such as improving quality of life, minimizing adverse effects. side effects of chemotherapy, radiation therapy and prolongation of life.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.