This is an automatically translated article.
The article was written by doctors of Internal Oncology Department, Vinmec Times City International General Hospital.Analytical results from the RAINBOW study provided a regimen of ramucirumab and paclitaxel for patients with gastric/gastroesophageal adenocarcinoma. This is considered a new advancement in the treatment of advanced gastric/gastro-oesophageal cancer.
1. Difficulty in treating stomach cancer today
Stomach cancer is the fifth most common malignancy in the world with 1 million new diagnoses, according to GLOBOCAN estimates. It is also the third leading cause of cancer death in both sexes globally.
Tumor removal surgery has the potential to cure stomach cancer in its early stages; however, 50-80% of patients still relapse after resection.
Most patients with gastric cancer are diagnosed at advanced stage or recur after surgery. Regardless of recent advances in diagnosis, surgery, or new treatments, the overall clinical outcome in patients with advanced gastric cancer remains modest. The 5-year survival rate for patients at this stage is only about 31%, many patients at the time of diagnosis the cancer has spread to other body parts.

Ung thư dạ dày là bệnh ác tính phổ biến thứ năm trên thế giới
2. Combination regimen of ramucirumab and paclitaxel for patients with gastric/gastro-oesophageal cancer
For patients with metastases, chemotherapy will be the primary weapon. If the tumor is Her-2 receptor positive, the patient also has a chance to be combined with an additional Her-2 anti-Her-2 drug, Trastuzumab. However, the percentage of Her-2-positive tumors is only about 25%. Many patients still fail this treatment or those whose tumors do not express Her-2 receptors.
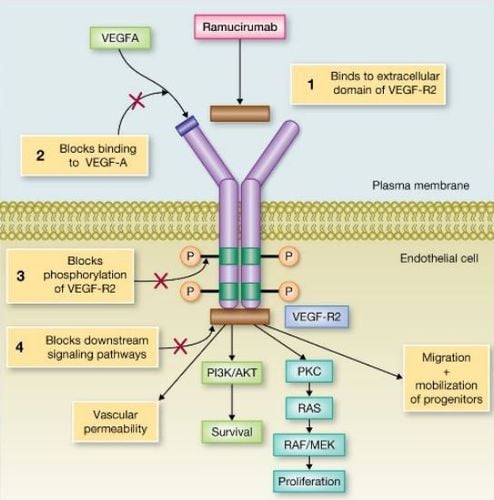
Ramucirumab and Trastuzumab are the only globally approved targeted therapies that target the HER-2 and VEGFR-2 receptors.
Ramucirumab Lilly Pharmaceuticals Cyramza is a human IgG1 monoclonal receptor antagonist, designed to bind to the extracellular domain of VEGFR-2, thereby preventing the binding of multiple ligands. VEGF and inhibit receptor activation.
Ramucirumab is approved by the US FDA, European Medical Association and other regulatory agencies for the treatment of gastric/gastro-oesophageal adenocarcinoma that has failed previous chemotherapy regimens. .
Ramucirumab in combination with paclitaxel is the preferred choice of many physicians worldwide for patients with metastatic gastric/gastroesophageal cancer who have failed previous treatment (chemotherapy with platinum- and fluoropyrimidine or trastuzumab in combination with cisplatin, 5-fluorouracil, capecitabine, etc.). The regimen is easy to implement, has few side effects and is highly effective.
RAINBOW was a study to evaluate the efficacy and safety of ramucirumab in combination with paclitaxel in a subgroup of patients with gastric/gastroesophageal carcinoma previously treated with trastuzumab.
Bệnh nhân ung thư dạ dày/ dạ dày- thực quản được điều trị phác đồ kết hợp ramucirumab và paclitaxel
How effective is the regimen of ramucirumab and paclitaxel in patients with gastric/gastroesophageal cancer?
Ramucirumab is the only biologic in combination with paclitaxel that has shown clinical benefit over chemotherapy alone in patients with advanced gastric cancer after failure of first-line chemotherapy. head. Ramucirumab has also been shown to be effective as a monotherapy in patients whose medical condition does not permit multidrug therapy or as maintenance therapy.
The study was conducted on 129 patients with stomach/gastroesophageal cancer, with a course of once every 2 weeks. RESULTS:
Median survival was longer in the ramucirumab and paclitaxel combination group (11.4 months; 95% CI: 7.0 -17.9) compared with placebo plus paclitaxel (7.0 months; 95% CI: 3.4 - 14.6); HR risk ratio: 0.68 (0.33 - 1.41); p = 0.30. Median progression-free survival was improved in the combination of ramucirumab and paclitaxel (4.2 months; 95% CI: 2.8 - 7.6) compared with placebo plus paclitaxel (2.7 months; 95% CI: 1.4 - 3.0). ; HR risk ratio: 0.30 (0.10 - 0.82); p = 0.01. Objective response and higher disease control were also seen in patients treated with ramucirumab in combination with paclitaxel compared with placebo and paclitaxel. The study concluded that the combination of ramucirumab and paclitaxel was effective in patients who had failed chemotherapy or chemotherapy + trastuzumab.
Always pioneering in applying the advances of science and technology, Vinmec Times City International Hospital is one of the first medical facilities in Vietnam to offer ramucirumab plus paclitaxel to patients. gastric/gastroesophageal adenocarcinoma. Initial results give high response, few side effects, suitable for elderly patients, poor physical condition.
For more information about the treatment of gastric/gastro-oesophageal cancer with the combined treatment regimen of ramucirumab and paclitaxel at Vinmec Times City, you can directly visit the Oncology Clinic in Central radiation oncology center , or contact HOTLINE: 0243 9743 556.
Reference source: Medscape