This is an automatically translated article.
The article was written by doctors of Internal Oncology - Vinmec Times City International General HospitalPembrolizumab (Keytruda) is one of a number of immunosuppressive cancer drugs. The following article briefly presents the results of a newly published study of Pembrolizumab (Keytruda) for advanced gastric and esophageal cancer.
1. Chemotherapy - The main treatment method for stomach cancer - oesophagus in the late stage
Stomach - esophageal cancer is one of the common cancers in our country. Although surgery plays a very important role in the treatment of this disease, unfortunately still a large proportion of patients are detected at a late stage, or the disease returns and distant metastases are no longer viable. surgical ability. At this time, chemotherapy is the mainstay of treatment. However, the results are still limited and especially the side effects of chemotherapy sometimes make it difficult for patients to pursue long-term. The discovery of a new drug, a new direction has been and is still being interested by scientists.
Pembrolizumab (Keytruda) is one of the drugs that treat cancer by immune mechanism. The drug is currently approved for initial treatment for some cancers such as lung cancer, melanoma ... with accompanying conditions such as the level of PD-L1 expression on the tumor. There is still a lot of research on this drug on different types of cancer, with different treatment steps.
2. Evaluation of the effectiveness of Pembrolizumab (Keytruda) in the treatment of gastric - esophageal cancer
The results of the randomized phase III study KEYNOTE-062 were presented at the ASCO Annual Meeting 2019 by Dr Josep Tabernero of the Vall debHebron University Hospital and the Institute of Cancer, Barcelona, Spain. According to Dr. Tabernero, the study showed that for patients with advanced gastrointestinal or gastroesophageal cancer, who were PD-L1-positive, HER2-negative, when receiving first-line therapy with pembrolizumab was given. survival outcomes were not inferior when compared with standard chemotherapy. Pembrolizumab showed a clinically significant improvement in patients with high PD-L1 expression. The study also evaluated the outcome of a combination of pembrolizumab and standard chemotherapy, but this option did not improve survival when compared with chemotherapy alone.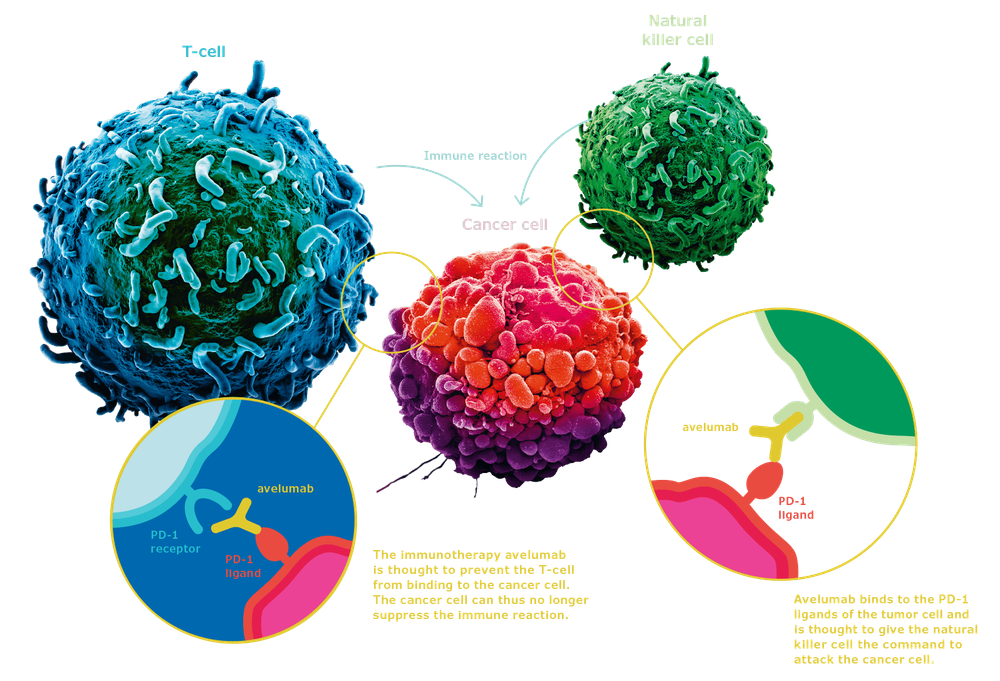
KEYNOTE062 (NCT02494583) was a randomized, controlled study. 763 Patients with tumors expressing PD-L1 ≥1%, HER2 negative, mean age 62 years and 26% having previous gastric surgery. Of these, 69% of the patients were diagnosed with gastric cancer and 30% had gastroesophageal cancer. Patients were randomly assigned to receive one of the following three options:
Either pembrolizumab monotherapy (256 patients) or pembrolizumab plus chemotherapy (257 patients) or chemotherapy alone (250 patients). ). The objective of the study was to compare progression-free survival (PFS) and overall response rate (ORR) of 3 groups, the relationship between response rate and PD-L1 expression level.
The results of the study show that, with an average follow-up time of 11.3 months
For patients with PD-L1 expression ≥1%: Pembrolizumab treatment group gave results not inferior to chemotherapy. Therapy For patients with PD-L1 expression ≥10%: Pembrolizumab alone gave better results than chemotherapy (17.4 months versus 10.8 months). But overall response rates were higher in the group that received both chemotherapy plus pembrolizumab The rate of grade 3 to 5 drug-related adverse events was 17% in the pembrolizumab group, 73% in the pembrolizumab plus chemotherapy group. data and 69% in the chemotherapy group
The authors concluded that: ''For advanced gastric and esophageal cancer, pembrolizumab in the first-line treatment was not inferior to that of pembrolizumab. with chemotherapy in patients with 1%-10% PD-L1 expression. For the PD-L1 group ≥ 10%, the improvement was clinically significant. The combination of pembrolizumab plus chemotherapy did not improve progression-free and overall survival in the group with PD-L1 = 1% - 10% ''.
Although the results of the study are still being analyzed to see which patients benefit most from pembrolizumab, there is hope nonetheless for the unfortunate patient with this disease. Especially those patients whose health is not suitable for chemotherapy.
Currently, Vinmec International General Hospital has also applied the results of the study into clinical practice, the initial results show that the patients tolerate it quite well, with few side effects.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.
Reference source: ESMO 2019