This is an automatically translated article.
The article was written by doctors of Internal Medicine Oncology, Vinmec Times City International General Hospital.Multiple clinical trials have now demonstrated immunotherapy with the PD-1 checkpoint inhibitor Keytruda (pembrolizumab) significantly improves survival when used as first-line treatment for carcinoma head and neck region with PD-L1 expression.
Final results of the KEYNote -048 clinical trial were announced at the annual meeting of the American Society of Clinical Oncology in Chicago, June 2019 and demonstrated superior survival compared with treatment. standard (Cisplatin and Cetuximab chemotherapy). KEYNOTE-048 has also confirmed that the PD-L1 CPS should be considered a routine test for head and neck cancers.
About head and neck cancer
Globally, head and neck cancer is the seventh most common cancer with an estimated 400,000-600,000 new diagnoses each year. Of these, the most likely histologically is squamous cell carcinoma.
Stage IV or recurrent, metastatic head and neck cancer include patients with a second lesion (in addition to the original tumor site) to other distant sites in the body. The 5-year survival rate for this group of patients is just under 4%, which shows the need for new research directions and new drugs to improve treatment outcomes.
Head and neck cancer originates in the tissues in or around the mouth, nose, and throat. Risk factors for head and neck cancer include smoking, alcohol consumption, and infection with high-risk types of human papillomavirus (HPV). Patients with head and neck cancer that have spread to other parts of the body or recurred after initial treatment have very few treatment options, so researchers continue to explore methods. new and more effective treatment for this type of cancer.

KEYNOTE-048 là xét nghiệm nên làm cho ung thư đầu và cổ
About Keytruda
Keytruda belongs to a new class of drugs called PD-1 inhibitors that have produced huge results increasing their ability to help the immune system recognize and attack cancer cells. PD-1 is a protein that inhibits certain types of immune responses, allowing cancer cells to evade an attack by certain immune cells in the body. Drugs that block the PD-1 pathway enhance the body's immune system's ability to fight cancer cells.
Keytruda in the study: KEYNOTE-048 Phase III clinical trial KEYNOTE-048 aimed to evaluate the therapeutic effect of Keytruda or Keytruda alone or Keytruda in combination with chemotherapy with a regimen including Cisplatin, Erbitux (Cetuximab) ) and 5-Fluorouracil (5-FU), which is the standard chemotherapy regimen for head and neck squamous cell carcinoma.
KEYTRUDA in combination with chemotherapy reduced the risk of death by 40% in patients with PD-L1-expressing tumors with CPS≥20, and extended survival to 14.7 months compared with 11 months for chemotherapy alone
In patients with PD-L1-expressing tumors with CPS 1 found that KEYTRUDA in combination with chemotherapy resulted in a 35% reduction in mortality risk and 3 months longer survival compared with chemotherapy alone. chemotherapy only. When used as monotherapy, KEYTRUDA produces a survival benefit similar to chemotherapy.
Keytruda has been approved for the treatment of patients with recurrent or metastatic head and neck cancer that has progressed on or after cisplatin-containing chemotherapy.
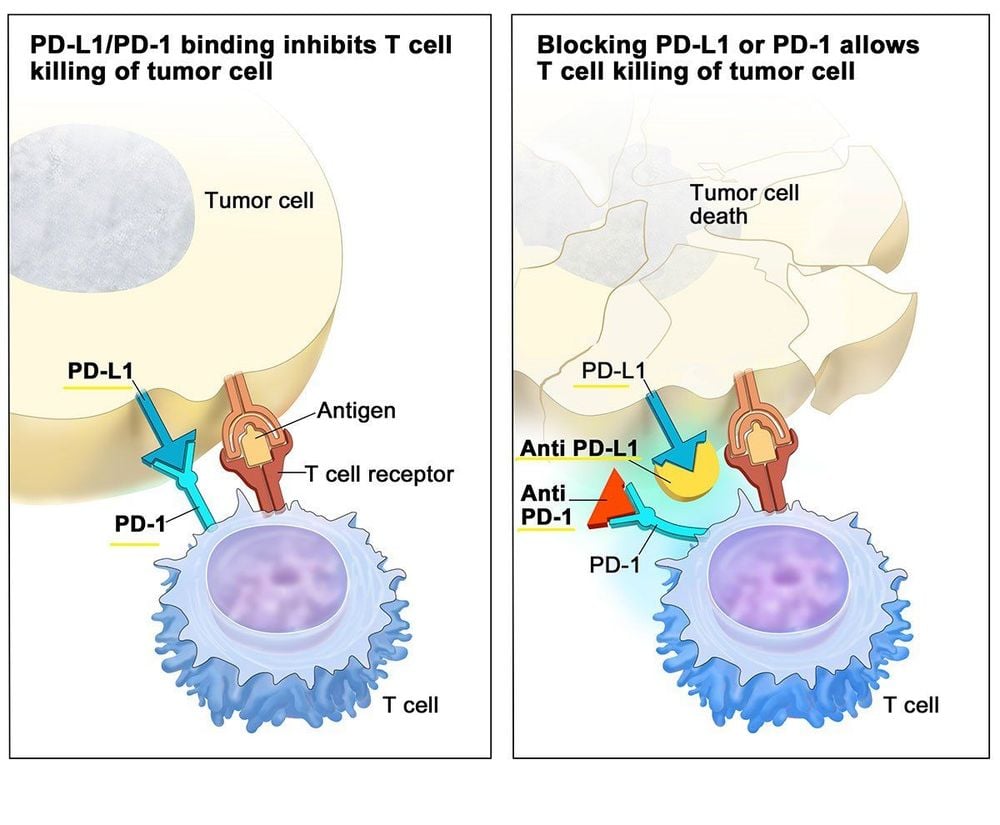
Keytruda ức chế PD-1 giúp tăng cường khả năng của hệ thống miễn dịch, chống lại tế bào ung thư
85% respond and persist for at least 6 months; 71% had a response and persisted for at least one year. At 6 months after initiating treatment with Keytruda, 58% of patients were alive and 25% of patients were alive with no cancer progression. At one year after initiating treatment with Keytruda, 38% of patients were alive and 17% of patients were alive with no cancer progression. Patients with good outcomes with Keytruda were followed for a median of 12.5 months at the time of data collection. Mean length of time that other treatment outcomes have not been achieved for this group of patients at this stage of the disease. Pembrolizumab (Keytruda) is generally well tolerated.

Keytruda hiện đang được chấp thuận để điều trị ung thư đầu và cổ giai đoạn tiến triển
Currently, VINMEC has applied this therapy to patients with head and neck squamous cell carcinoma. Initial results are positive and many patients are elderly but well tolerated. Depending on the location of the lesion, we combine it with local and regional thermogenic therapy.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.