This is an automatically translated article.
The article was written by MSc Ma Van Tham - Internal Medicine Doctor, Department of Pediatrics - Neonatology - Vinmec Phu Quoc International General Hospital.
Cytomegalovirus was first noticed in 1881 by Ribbert H. He discovered enlarged cells in the renal tissue of a patient with congenital syphilis and in the parotid gland for unknown reasons until he Read the report of Jesionek and Kiolemenoglou to get an explanation.
4. General pathophysiology of cytomegalovirus
Cytomegalovirus is a member of the family of 8 Herpesviruses that cause disease in humans, such as herpesvirus 5 (HHV-5). CMV is referred to as a Betaherpesvirinae, based on its propensity to infect monocytes and lymphocytes.
Cytomegalovirus is the largest member of the herpesvirus family, with a double-stranded DNA genome of more than 240 kbp, capable of encoding more than 200 potential protein products. The function of most of these proteins remains unclear. As with other herpesviruses, the structure of the viral particle is an icosahedral capsid, surrounded by a lipid envelope outside the bilayer.
An understanding of viral replication provides insights into the molecular mechanisms of antiviral therapy and protective immunity. Cytomegalovirus replicates very slowly in cell culture, reflecting its pattern of very slow growth in vivo (in contrast to herpes simplex HSV virus infection, which progresses very rapidly). The cytomegalovirus replication cycle is transiently divided into the following three regulatory classes: immediate, early, and late.
Immediate gene duplication occurs during the first 4 hours after viral infection, when key regulatory proteins that allow the virus to control the cellular machinery are synthesized. The major activator for this process in the CMV genome is one of the most potent eukaryotic promoters described in nature, which has been discovered in modern biotechnology as a useful activator. for gene expression in gene therapy and vaccination studies.
Following the immediate synthesis of the gene, the gene products are soon transcribed. Initial gene products include copies of DNA proteins and some structural proteins.
Finally, late gene products are made about 24 h after infection, and these proteins are mainly structural proteins involved in virion assembly and units.
Synthesis of terminal genes is highly dependent on viral DNA replication and can be blocked by viral DNA polymerase inhibitors, such as Ganciclovir. The lipid bilayer contains the encoded viral glycoprotein, which is the primary target of the host's humoral response. These glycoproteins are the building blocks of vaccines. The proteinaceous layer between the envelope and the inner capsid, the viral envelope, contains proteins that are the primary targets of the cell-mediated immune response. The most important of these envelope proteins is the so-called large envelope protein, UL83 (phosphoprotein 65 [pp65]).
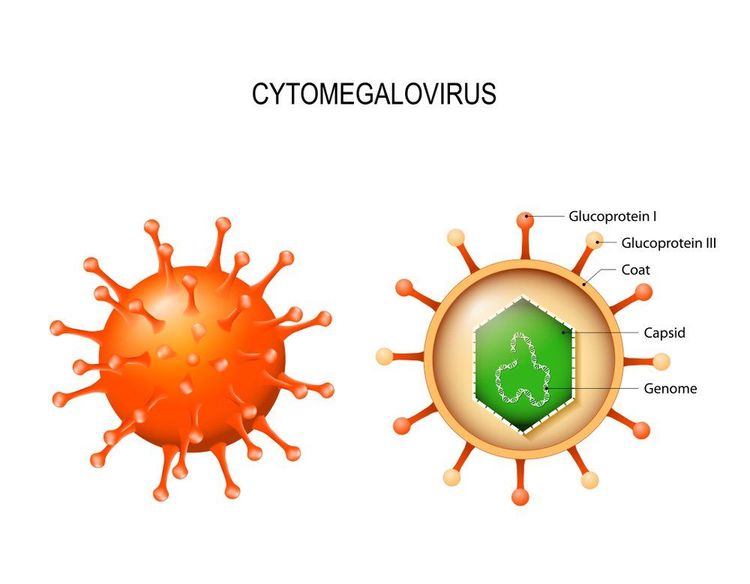
Clinically, one of the manifestations of Cytomegalovirus infection is cytomegalic cells. The enlarged infected cells (from "Cytomegaly", a compound of the virus's name) contain the intranuclear substance seen on the histopathology of owls' eyes. The presence of these cells indicates an advanced infection, although it may not be present even in strongly infected tissues. In most cell lines, Cytomegalovirus is difficult to culture in the laboratory, however, in vivo infection seems to involve mainly epithelial cells. In severe cytomegalovirus disease, involvement can be observed in most organ systems.
Understanding of the molecular mechanisms in the pathogenesis of CMV tissue damage is limited, especially in congenital CMV infection. Although the central nervous system is the major organ of injury during fetal development, finding CMV from the cerebrospinal fluid of neonates with congenitally infected symptoms is quite difficult.
Because CMV can infect endothelial cells, some authors postulated that a viral angitis may be responsible for the cerebral hypoperfusion that causes growth retardation. Others argue that CMV is directly teratogenic in the fetus. The observation of CMV alternation in the cell cycle and CMV-induced chromosomal injury supported this study, however, this hypothesis was difficult to test experimentally.
5. Immune response
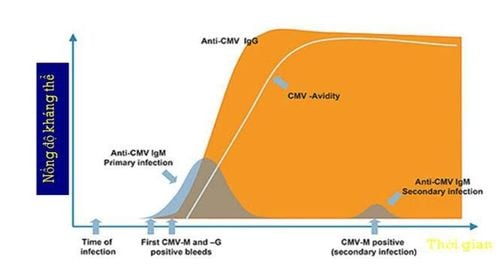
Primary CMV infection is defined when infection in an individual must be confirmed to be seronegative for CMV. In these patients, CMV immunoglobulin M (IgM) antibodies can be detected as early as 4-7 weeks after initial infection and may persist for 16-20 weeks.
CMV IgG antibodies persist from infection to the rest of life. Most neutralizing antibodies are directed against the envelope of Glycoprotein gB. Studies have shown that more than 50% of the neutralizing activity with serum neutrophils is related to the gB glycoprotein. However, virion microencapsulated proteins such as pp150, pp28 and pp65 are strongly expressed and antibody responses are stable.
CMV is an immunomodulatory virus and can aggravate an existing immune condition (such as lupus erythematosus).
CMV DNA in the blood and virus in the blood are commonly found in healthy female individuals with CMV (+). Natural acquired immunity to the virus does not appear to prevent reinfection or the process by which the virus hides in the body.
Cell mediated immunity is considered to be the most important factor in fighting CMV infection. Patients with deficient cell-mediated immunity are at greatest risk for CMV disease.
CD4+ and CD8+ specific lymphocytes play an important role in immune defense after primary infection or reactivation of an underlying disease.
Studies of bone marrow transplant recipients have shown no formation and proliferation of CMV-specific CD4+ or CD8+ cells in the highest risk group for CMV pneumonia.
In addition, no cases of CMV pneumonia have been reported in allogeneic marrow transplant recipients receiving CMV-specific CD8+ infusions.
Vinmec International General Hospital is one of the hospitals that not only ensures professional quality with a team of leading medical doctors, a system of modern equipment and technology. The hospital provides comprehensive and professional medical examination, consultation and treatment services, with a civilized, polite, safe and sterile medical examination and treatment space. Customers when choosing to perform tests here can be completely assured of the accuracy of test results.
Please dial HOTLINE for more information or register for an appointment HERE. Download MyVinmec app to make appointments faster and to manage your bookings easily.